amesergide
- CAS NO.:121588-75-8
- Empirical Formula: C25H35N3O
- Molecular Weight: 393.56
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
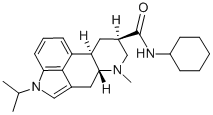
What is amesergide?
Originator
Amesergide,Onbio Inc.
The Uses of amesergide
Serotonin antagonist.
Manufacturing Process
To a 250 ml three-neck round bottom flask was added 10.0 g (32.01 mmol) of
(8β)-1-isopropyl-6-methylergoline-8-carboxylie acid, 4.43 g (32.1 mmol) of
potassium carbonate and 200 ml of N,N-dimethylformamide. The mixture was
refluxed and 25 ml of a distillate was collected. The remaining solution was
cooled in an ice bath, and then with an acetonitrile/carbon dioxide bath which
lowered the temperature of the reaction mixture to about -45°C this mixture
was added 4.59 g (33.62 mmol) of isobutyl chloroformate dropwise. The
resulting mixture was stirred for approximately 5 min and 3.49 g (35.21
mmol) of cyclohexylamine was added. The reaction mixture was allowed to
warm to room temperature and stirred for approximately 19 h. To the mixture
was added 500 ml of ice water containing 25 ml of concentrated ammonium
hydroxide. The mixture was cooled and the precipitated solid was collected by
vacuum filtration. The resulting solid was washed with water and dried in
vacuo to provide 10.13 g (yield 76.8%) of the (8β)-N-cyclohexyl-1-isopropyl-
6-methylergoline-8-carboxamide having a purity of 92.3%.
The resulting solid was combined with three other lots of the desired
compound previously synthesized to provide a total weight of 33.6 g. This
material was dissolved in 1200 ml of hot methanol and the resulting solution
was filtered. The filtrate was allowed to cool to room temperature and 600 ml
of water was added dropwise. The mixture was cooled in the freezer and the
precipitated crystals were collected by vacuum filtration. The crystals were
washed with methanol and dried in vacuo to provide 26.95 g of the desired
compound having a purity of 96.5% as determined by HPLC. The dried solid
was dissolved in 1100 ml of hot methanol, and the resulting solution was
filtered hot and allowed to cool. To this mixture was added 600 ml of water
and again the precipitated solid was collected by vacuum filtration. The solid
was washed with water and dried in vacuo to provide 25.82 g of the (8β)-Ncyclohexyl-
1-isopropyl-6-methylergoline-8-carboxamide, melting point 250°C.
The assayed material indicated 98.7% purity.
Therapeutic Function
Serotonin antagonist
Safety information for amesergide
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 3-Nitrobenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1