Amentoflavone
Synonym(s):Didemethyl-ginkgetin;I3′,II8-Biapigenin;Tridemethylsciadopitysin
- CAS NO.:1617-53-4
- Empirical Formula: C30H18O10
- Molecular Weight: 538.46
- MDL number: MFCD00017470
- EINECS: 000-000-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
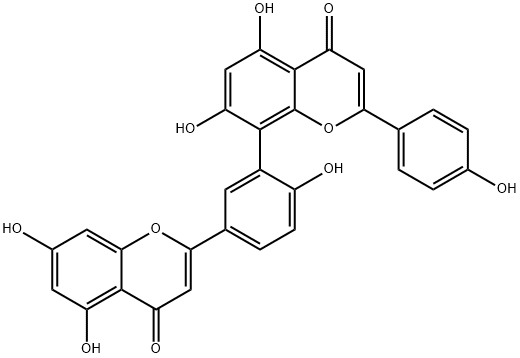
What is Amentoflavone?
Description
Amentoflavone is a biflavonoid originally isolated from Selaginella. It has a wide variety of biological effects including antibacterial, antioxidant, antiviral, antidiabetic, and neuroprotective activities. Amentoflavone has antiviral activity against the influenza A subtypes H1N1 and H3N2, influenza B, and herpes simplex virus 1 (EC50s = 3.1 and 4.3, 0.56, and 17.9 μg/ml, respectively). It has antidiabetic effects such that it dose-dependently increases insulin receptor phosphorylation and activation and inhibits hydrolysis of p-nitrophenyl phosphate (p-NPP) catalyzed by protein tyrosine phosphatase 1B (PTP1B; IC50 = 7.3 μM). Amentoflavone reduces the time mice spend immobile in the forced swim test, a measure of antidepressant efficacy, in a dose-dependent manner.
Description
Amentoflavone is a mild antidepressant found in many plants, such as?Hypericum perforatum?L. (St. John''s wort). It has also been shown to exhibit antiviral and anticancer activity and is a potent antioxidant.
The Uses of Amentoflavone
Amentoflavone, is isolated from an Et acetate ext. of the whole plant of Selaginella tamariscina. It has been shown to have antitumor activity, such as mitochondria-mediated apoptotic cell death. Amentoflavone can interact with many medications by being a potent inhibitor of CYP3A4 and CYP2C9, which are enzymes responsible for the metabolism of some drugs in the body.
What are the applications of Application
Amentoflavone is an anti-viral and anti-inflammatory agent that up-regulates PPAR-γ
Definition
ChEBI: Amentoflavone is a biflavonoid that is obtained by oxidative coupling of two molecules of apigenin resulting in a bond between positions C-3 of the hydroxyphenyl ring and C-8 of the chromene ring. A natural product found particularly in Ginkgo biloba and Hypericum perforatum. It has a role as a cathepsin B inhibitor, an antiviral agent, an angiogenesis inhibitor, a P450 inhibitor and a plant metabolite. It is a biflavonoid, a hydroxyflavone and a ring assembly.
Biochem/physiol Actions
Biflavonoid with anti-inflammatory, anti-viral and cancer chemopreventive activity. It inhibits vascularization of tumors by blocking the activity of angiogenic VEGFs. Blocks the induction of COX-2 and up-regulates PPAR-γ. It is a negative modulator of the GABAA receptor at the benzodiazepine binding site.
Properties of Amentoflavone
| Melting point: | >300°C (dec.) |
| Boiling point: | 910.5±65.0 °C(Predicted) |
| Density | 1.656±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated), Pyridine (Slightly) |
| form | neat |
| pka | 6.01±0.40(Predicted) |
| form | Solid |
| color | Yellow |
| BRN | 380244 |
| CAS DataBase Reference | 1617-53-4(CAS DataBase Reference) |
Safety information for Amentoflavone
Computed Descriptors for Amentoflavone
| InChIKey | YUSWMAULDXZHPY-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Amentoflavone CAS 1617-53-4View Details
Amentoflavone CAS 1617-53-4View Details
1617-53-4 -
 Amentoflavone CAS 1617-53-4View Details
Amentoflavone CAS 1617-53-4View Details
1617-53-4 -
 Amentoflavone CAS 1617-53-4View Details
Amentoflavone CAS 1617-53-4View Details
1617-53-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1