Ambrisentan
Synonym(s):(+)-(2S)-2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropanoic acid
- CAS NO.:177036-94-1
- Empirical Formula: C22H22N2O4
- Molecular Weight: 378.42
- MDL number: MFCD09842330
- EINECS: 658-059-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
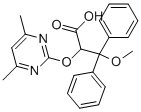
What is Ambrisentan?
Absorption
Ambrisentan is rapidly absorbed with peak plasma concentrations occuring around 2 hours after oral administration. Cmax and AUC increase proportionally with dose across the therapeutic dosing range. Absolute oral bioavailability of ambrisentan is unknown. Absorption is not affected by food.
Toxicity
Ambrisentan is teratogenic and has a high risk of embryo-fetal toxicity. LD50 was found to be greater than or equal to 3160 mg/kg when studied in rats. There was no evidence of carcinogenic potential in 2 year oral daily dosing studies in rats and mice.
Description
Ambrisentan is a selective endothelin-A (ETA) receptor antagonist introduced for the oral treatment of patients with pulmonary arterial hypertension (PAH), to improve exercise capacity and delay clinical worsening. It is the third ET-receptor antagonist to be marketed for this indication behind bosentan and sitaxsentan. PAH is a rare disease of the small pulmonary arteries characterized by vascular proliferation and remodeling, resulting in a progressive increase in pulmonary vascular resistance and pulmonary arterial pressure, and ultimately, right ventricular failure and premature death. Early symptoms of PAH include gradual onset of shortness of breath, fatigue, palpitation, edema, and fainting. Endothelin-1 (ET-1), a potent vasoconstrictor and smooth muscle mitogen, is a key contributor to the acceleration of the disease, and its effects are mediated through activation ofETA and ETB receptors.
Description
Ambrisentan is a nonpeptide endothelin A (ETA) receptor antagonist (IC50s = 0.251, 0.316, 0.398, 251, and 630 nM for rat preparations of heart, bladder, kidney, lung, and cerebral cortex, respectively). It inhibits contraction of isolated rabbit aortic rings induced by endothelin-1 (ET-1; ) by 43.23% when used at a concentration of 1 μM. Ambrisentan inhibits ET-1-induced contraction of human pulmonary and radial arteries in vitro (Kd = 0.042 and 0.11 μM, respectively). In a rat model of neonatal hyperoxic lung injury, ambrisentan (20 mg/kg per day, s.c.) reduces pulmonary arterial hypertension (PAH) as well as decreases PAH-induced right ventricular hypertrophy (RVH) and peak RV pressure. Formulations containing ambrisentan have been used to treat PAH.
Chemical properties
White to Off White Solid
Originator
Abbott (US)
The Uses of Ambrisentan
Nonpeptide endothelin ETA receptor antagonist. Antihypertensive
The Uses of Ambrisentan
antihypertensive;endothelin receptor antagonist
What are the applications of Application
Ambrisentan is a nonpeptide endothelin ETAR (ETA receptor) antagonist and antihypertensive
Background
Ambrisentan is an orally active selective type A endothelin receptor antagonist indicated for the treatment of pulmonary arterial hypertension. It is approved in Europe, Canada and the United States for use as a single agent to improve exercise ability and delay clinical worsening. In addition, it is approved in the United States for use in combination with tadalafil to reduce the risks of disease progression, hospitalization and to improve exercise ability. Studies establishing the efficacy of Ambrisentan included patients with both idiopathic or heritable pulmonary arterial hypertension and those with pulmonary arterial hypertension associated with connective tissue diseases. Patients studied displayed symptoms and etiologies predominantly of WHO Functional Class II-III. As an endothelin receptor antagonist, Ambrisentan prevents endogenous endothelin peptide from constricting the muscles in blood vessels, allowing them to relax and permit a reduction in blood pressure.
Indications
Ambrisentan is indicated for treatment of idiopathic (‘primary’) pulmonary arterial hypertension (IPAH) and pulmonary arterial hypertension (PAH) associated with connective tissue disease in patients with WHO functional class II or III symptoms. In the United States of America, ambrisentan is also indicated in combination with tadalafil to reduce the risks of disease progression and hospitalization for worsening PAH, and to improve exercise ability.
Definition
ChEBI: Ambrisentan is a diarylmethane.
brand name
Letairis
General Description
Ambrisentan, (+)-(2S)-2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropanoic acid(Letairis), is a potent ETA selective endothelin antagonist that,is indicated, in the treatment of pulmonary arterial hypertension(PAH). PAH is a rare disease that if left untreated has ahigh mortality rate. In June of 2007, the FDA granted approvalof ambrisentan for once-daily treatment of PAH.Studies have shown that it improves a 6-minute walk by about30 to 60 m for patients receiving placebo.
Pharmacokinetics
Ambrisentan 10 mg daily had no significant effect on the QTc interval, whereas a 40 mg daily dose of ambrisentan increased mean QTc at tmax by 5 ms with an upper 95% confidence limit of 9 ms. Significant QTc prolongation is not expected in patients taking ambrisentan without concomitant metabolic inhibitors. Plasma concentrations of B-type natriuretic peptide (BNP) in patients who received ambrisentan for 12 weeks were significantly decreased. Two Phase III placebo-controlled studies demonstrated a decrease in BNP plasma concentrations by 29% in the 2.5 mg group, 30% in the 5 mg group, and 45% in the 10 mg group (p < 0.001 for each dose group) and an increase by 11% in the placebo group.
Clinical Use
Endothelin A (ETA) receptor antagonist:
Treatment of pulmonary arterial hypertension
Synthesis
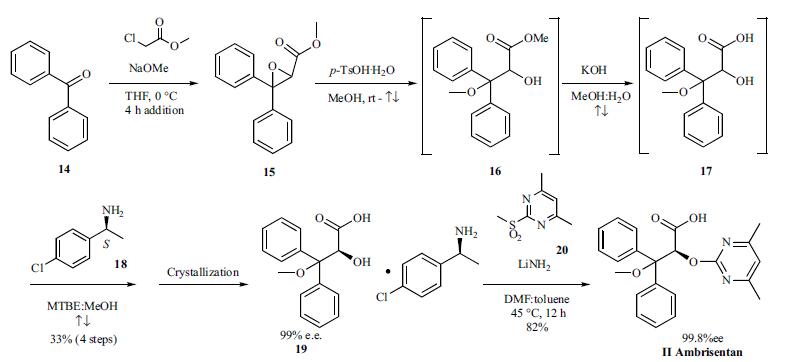
Both the discovery and process routes to the synthesis of ambrisentan have been published and the process route is described as shown in the scheme. Reacting a mixture of benzophenone (14) and sodium methoxide in THF at 0??C with methylchloroacetate over a four hour period provided glycidate 15 which was taken forward without purification to the subsequent step. Addition of ptoluenesulfonic acid monohydrate to a solution of glycidate 15 in methanol was followed by heating at reflux and distilling out the solvent until the temperature reached 66??C. While the solution was still refluxing, 10% potassium hydroxide was added and the remaining organic solvent was distilled out until the temperature reached 94??C, providing complete hydrolysis to acid 16. The reaction was cooled to room temperature and diluted with water and methyl tert-butylether (MTBE) then acidified with 10% sulfuric acid. The MTBE layer was separated and taken to the next step. Additional MTBE and methanol were added to the crude acid 17 and the resulting mixture was heated at reflux. (S)-1-(4-chlorophenyl) ethylamine was added to the refluxing solution and the resulting mixture was allowed to cool to 0-5??C slowly at a rate of 10??C/h which resulted in crystallization of the salt 19 in 33% overall yield from benzophenone and 99% e.e. The chiral hydroxyl acid salt 19 was mixed with sulfone 20 and lithium amide in a toluene/DMF mixture and heated at 45??C for 12 hours to give, after acidic workup and crystallization, ambrisentan (II) in 84% yield as a colorless powder with 99.8% e.e.
Drug interactions
Potentially hazardous interactions with other drugs
Ciclosporin: concentration of ambrisentan doubled
with an increased risk of side effects; maximum dose
5 mg daily.
Metabolism
Ambrisentan is a metabolized primarily by uridine 5’-diphosphate glucuronosyltransferases (UGTs) 1A9S, 2B7S,1A3S to form ambrisentan glucuronide. Ambrisentan is also metabolized to a lesser extent by CYP3A4, CYP3A5 and CYP2C19 to form 4- hydroxymethyl ambrisentan which is further glucuronidated to 4-hydroxymethyl ambrisentan glucuronide.
Metabolism
Ambrisentan is glucuronidated via several UGT
isoenzymes (UGT1A9S, UGT2B7S and UGT1A3S)
to form ambrisentan glucuronide (13%). Ambrisentan
also undergoes oxidative metabolism mainly by CYP3A4
and to a lesser extent by CYP3A5 and CYP2C19 to form
4-hydroxymethyl ambrisentan (which has little activity)
which is further glucuronidated to 4-hydroxymethyl
ambrisentan glucuronide.
Ambrisentan is excreted mainly by the liver, although the
contribution of hepatic metabolism and biliary excretion
is unknown.
Storage
Store at +4°C
References
[1] vatter h, seifert v. ambrisentan, a non-peptide endothelin receptor antagonist. cardiovascular drug reviews, 2006, 24(1): 63-76.
[2] barst r j. a review of pulmonary arterial hypertension: role of ambrisentan. vascular health and risk management, 2007, 3(1): 11.
Properties of Ambrisentan
| Melting point: | >150°C (dec.) |
| Boiling point: | 551.1±60.0 °C(Predicted) |
| Density | 1.228±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 0.97±0.10(Predicted) |
| color | White to Off-White |
| Merck | 14,384 |
| InChI | InChI=1S/C22H22N2O4/c1-15-14-16(2)24-21(23-15)28-19(20(25)26)22(27-3,17-10-6-4-7-11-17)18-12-8-5-9-13-18/h4-14,19H,1-3H3,(H,25,26) |
Safety information for Ambrisentan
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Ambrisentan
| InChIKey | OUJTZYPIHDYQMC-UHFFFAOYSA-N |
| SMILES | C(OC1N=C(C)C=C(C)N=1)(C(O)=O)C(OC)(C1=CC=CC=C1)C1=CC=CC=C1 |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





You may like
-
 Ambrisentan 99%View Details
Ambrisentan 99%View Details -
 177036-94-1 98%View Details
177036-94-1 98%View Details
177036-94-1 -
 Ambrisentan 177036-94-1 98%View Details
Ambrisentan 177036-94-1 98%View Details
177036-94-1 -
 177036-94-1 98%View Details
177036-94-1 98%View Details
177036-94-1 -
 Ambrisentan 177036-94-1 99%View Details
Ambrisentan 177036-94-1 99%View Details
177036-94-1 -
 Ambrisentan CAS 177036-94-1View Details
Ambrisentan CAS 177036-94-1View Details
177036-94-1 -
 Ambrisentan 98% CAS 177036-94-1View Details
Ambrisentan 98% CAS 177036-94-1View Details
177036-94-1 -
 Ambrisentan CAS 177036-94-1View Details
Ambrisentan CAS 177036-94-1View Details
177036-94-1
