Aluminium phosphate
Synonym(s):Aluminum monophosphate;Monoaluminum phosphate
- CAS NO.:7784-30-7
- Empirical Formula: AlO4P
- Molecular Weight: 121.95
- MDL number: MFCD00003430
- EINECS: 232-056-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
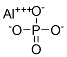
What is Aluminium phosphate?
Description
Aluminum phosphate is naturally found in the mineral beryl. It is also a component of several other minerals, including phosphate rock, grossular, azurite, and phosphate rock. Its industrial uses include its use as a ceramic flux, as a cement component when mixed with calcium sulfate and sodium silicate, and in the production of specialty glasses. Furthermore, it can be formulated into a drying gel for use as an antacid in treatments.
Chemical properties
Aluminum phosphate adjuvant is a white hydrogel that sediments slowly and forms a clear supernatant.
Physical properties
White powdery solid (rhombic plate); the mineral berlinite (AlPO4) has hexagonal quartz-like structure; refractive index 1.546; mp > 1,500°C; density 2.566 g/cu3; insoluble in water and alcohol; Ksp 9.83x10-10 very slightly soluble in HCl or HNO3.
The Uses of Aluminium phosphate
Aluminium phosphate has diverse applications across several industries. It functions as a flux for ceramics and is utilized in the manufacture of specialized glasses. In construction and dentistry, it is employed in the preparation of dental cement and as a component in cement formulations with calcium sulfate and sodium silicate. Chemically, it serves as a catalyst and a molecular sieve. Pharmaceutical applications include its use as a gel. Furthermore, it is incorporated into emollients and fire-resistant coatings, and acts as an anti-fouling agent in the textile industry.
Production Methods
Aluminum phosphate adjuvant is formed by the reaction of a solution of aluminum chloride and phosphoric acid with alkali hydroxide.
Preparation
It is prepared by treating sodium aluminate with phosphoric acid.
brand name
Phosphaljel (Wyeth-Ayerst).
General Description
A colorless liquid. Insoluble in water. Corrosive to metals and tissue.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
An acid. The resulting aqueous solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
Solutions are corrosive to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Aluminum phosphate adjuvant is used in parenteral human and veterinary vaccines.It activates Th2 immune responses, including IgG and IgE antibody responses.
Clinical Use
Phalu Gel's main active ingredient is aluminum Phosphorus. Aluminum Phosphate works to reduce excess stomach acid. This gel suspension, when taken to the stomach, will form a protective layer on the surface of the gastrointestinal mucosa, similar to the effect of mucus or mucosal protective factors to help cover the stomach from the impact of fluids. The aluminum phosphate layer in the stomach has a smooth dispersion effect, going to the ulcers in the stomach and making them heal faster, making the patient feel more comfortable and less painful. Aluminum Phosphorus, when used, does not affect the intestinal absorption of Phosphorus. Phalu-Gel will quickly reduce the pain of stomach ulcers and the discomfort of excess acid, help heal the ulcers quickly, and make the patient feel comfortable right away.Aluminum hydroxide, aluminum phosphate, and potassium aluminum sulfate (alum) are used as adjuvants in vaccines designed to stimulate systemic immunity. Aluminum salts are used in DPT, pneumocccal conjugate, hepatitis A, papilloma, anthrax, and rabies vaccines. Like other systemic adjuvants, they form an insoluble depot and slowly release the antigen, which stimulates an antibody response.
Safety Profile
Corrosive to the eyes, skin, and mucous membranes. When heated to decomposition it emits toxic fumes of POx. Used as an antacid and as a cement component, flux for ceramics, dental cement, glass, and gels. See also ALUMINUM COMPOUNDS and PHOSPHATES
Safety
Aluminum phosphate adjuvant is intended for use in parenteral vaccines and is generally regarded as safe. It may cause mild irritation, dryness, and dermatitis on skin contact. It may also cause redness, conjunctivitis, and short-term mild irritation on eye contact. Ingestion of large amounts of aluminum phosphate adjuvant may cause respiratory irritation with nausea, vomiting, and constipation. Inhalation is unlikely, although the dried product may cause respiratory irritation and cough. Type I hypersensitivity reactions following parenteral administration have also been reported.
Potential Exposure
Used as a flux in ceramics; in dental cements; in the manufacture of special glasses, paints and varnishes, cosmetics; making pulp and paper; as an antacid.
Metabolism
Not Available
Storage
Aluminum phosphate adjuvant is stable for at least 2 years when stored at 4–308℃ in well-sealed inert containers. It must not be allowed to freeze as the hydrated colloid structure will be irreversibly damaged.
Shipping
UN1760 Corrosive liquids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN3260 Corrosive solid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.
Incompatibilities
A strong oxidizer; keep away from combustible materials. Violent reaction with reducing agents; strong bases. Material is an inorganic acid and will react, possibly violently, with bases; corrosive to metals, some plastics and body tissues
Incompatibilities
The point of zero charge is related directly to the Al : P atomic ratio. Therefore, the substitution of additional phosphate groups for hydroxyl groups will lower the point of zero charge. Substitution of carbonate, sulfate, or borate ions for hydroxyl groups will also lower the point of zero charge.
Regulatory Status
GRAS listed. Accepted for use in human and veterinary vaccines in Europe and the USA. The limits for use in human vaccines are 0.85 mg aluminum/dose (FDA) and 1.25 mg aluminum/dose (WHO). There are no established limits for use in veterinary vaccines. Reported in the EPA TSCA Inventory.
Properties of Aluminium phosphate
| Melting point: | 1500 °C |
| Density | 2.56 g/mL at 25 °C (lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Very slightly soluble in water, practically insoluble in alcohol. It dissolves in dilute solutions of mineral acids and alkali hydroxides. |
| form | Powder |
| color | White |
| Specific Gravity | 2.566 |
| Water Solubility | Insoluble |
| Merck | 14,358 |
| Solubility Product Constant (Ksp) | pKsp: 20.01 |
| Exposure limits | ACGIH: TWA 1 mg/m3 |
| Dielectric constant | 6.0(Ambient) |
| InChI | InChI=1S/Al.H3O4P/c;1-5(2,3)4/h;(H3,1,2,3,4)/q+3;/p-3 |
| CAS DataBase Reference | 7784-30-7(CAS DataBase Reference) |
| EPA Substance Registry System | Monoaluminum phosphate (7784-30-7) |
Safety information for Aluminium phosphate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Aluminium phosphate
| InChIKey | ILRRQNADMUWWFW-UHFFFAOYSA-K |
| SMILES | [Al+3].P([O-])([O-])([O-])=O |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

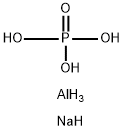
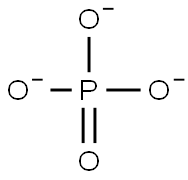
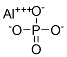
You may like
-
 Aluminum phosphate, tribasic 98%View Details
Aluminum phosphate, tribasic 98%View Details -
 Aluminum phosphate CAS 7784-30-7View Details
Aluminum phosphate CAS 7784-30-7View Details
7784-30-7 -
 Aluminum phosphate CAS 7784-30-7View Details
Aluminum phosphate CAS 7784-30-7View Details
7784-30-7 -
 Aluminum phosphate CAS 7784-30-7View Details
Aluminum phosphate CAS 7784-30-7View Details
7784-30-7 -
 Aluminum phosphate CAS 7784-30-7View Details
Aluminum phosphate CAS 7784-30-7View Details
7784-30-7 -
 Aluminium phosphate CAS 7784-30-7View Details
Aluminium phosphate CAS 7784-30-7View Details
7784-30-7 -
 Aluminum phosphate CAS 7784-30-7View Details
Aluminum phosphate CAS 7784-30-7View Details
7784-30-7 -
 Aluminum phosphate CAS 7784-30-7View Details
Aluminum phosphate CAS 7784-30-7View Details
7784-30-7
