phosphate
- CAS NO.:264888-19-9
- Empirical Formula: O4P-3
- Molecular Weight: 94.97
- MDL number: MFCD00145108
- Update Date: 2023-11-28 16:31:44
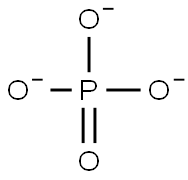
What is phosphate?
Description
Excess of phosphate can lead to interactions within the human body with calcium, iron and magnesium, and
can lead to diarrhoea and may even be toxic. Phosphate and calcium levels are directly connected, and an
excess of phosphate will lead to the removal of calcium from the bones and teeth. This will cause osteoporosis
and problems with the health of teeth and gums. Athletes often use phosphate supplementation, but a
healthcare specialist should monitor this application.
Interactions for phosphate preparations with several over-the-counter and prescription drugs are known.
Antacids containing aluminium, calcium and magnesium ions can bind phosphate in the digestive tract and
prevent phosphate from being absorbed. This can lead in extreme cases to hypophosphataemia. Potassiumsparing
diuretics and potassium supplements in combinationwith phosphate preparations may lead to elevated
levels of blood potassium levels (hyperkalaemia). Hyperkalaemia can be a serious life-threatening problem.
The Uses of phosphate
Any salt of phosphoric acid. The salts include disodium phosphate, trisodium phosphate, sodium hexametha, and others. They play a variety of roles such as sequestrants, emulsifiers, solubility enhancers, and buffers in a variety of foods.
The Uses of phosphate
Phosphate, Ion chromatography standard solution is used as a standard solution in analytical chemistry and in ion chromatography. It is used for calibration of ion chromatography and other analytical techniques.
Veterinary Drugs and Treatments
Phosphate is useful in large volume parenteral fluids to correct or prevent hypophosphatemia when adequate oral phosphorous intake is not possible. Hypophosphatemia may cause hemolytic anemia, thrombocytopenia, neuromuscular and CNS disorders, bone and joint pain, and decompensation in patients with cirrhotic liver disease. There is some controversy whether “a low phos” indicates that treatment is necessary.
Properties of phosphate
| form | Liquid |
| color | Clear |
| Water Solubility | Miscible with water. |
| Dielectric constant | 4.0(Ambient) |
Safety information for phosphate
Computed Descriptors for phosphate
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneYou may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
