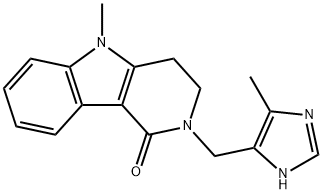
Alosetron hydrochloride
Synonym(s):2,3,4,5-Tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-1H-pyrido[4,3-b]indol-1-one hydrochloride
- CAS NO.:122852-69-1
- Empirical Formula: C17H19ClN4O
- Molecular Weight: 330.81
- MDL number: MFCD03453647
- EINECS: 802-450-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
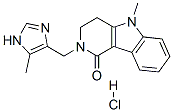
What is Alosetron hydrochloride?
Description
Lotronex (alosetron) was developed for the treatment of severe
irritable bowel syndrome (IBS), and was approved for use by
the US Food and Drug Administration (FDA) in 2000.
Although Lotronex had a relatively high improvement rate in
patients taking the drug for IBS, it was voluntarily pulled from
the market by GlaxoWellcome that same year due to reports of
severe adverse side effects, some resulting in death.
In 2002, Lotronex was reapproved in a supplemental New
Drug Application for use under more restrictive conditions.
Now with a risk management program to be consulted prior to
administration of the drug, Lotronex is designated to be
prescribed only when its medical benefits outweigh the risks of
toxic effects; women with severe diarrhea-predominant IBS are
now the focal point of prescriptions for Lotronex.
Chemical properties
Alosetron hydrochloride is Crystalline Solid
Originator
Alosetron hydrochloride,GlaxoSmithKline
The Uses of Alosetron hydrochloride
Alosetron hydrochloride is used in treatment of irritable bowel syndrome
The Uses of Alosetron hydrochloride
Lotronex is used for severe diarrhea-predominant IBS in women. There are other potential uses, as animal models have shown at least some evidence for the ability of Lotronex to mitigate the effects of psychosis, anxiety, cognitive impairment, emesis, and drug withdrawal. These possibilities have not been verified in humans, however.
What are the applications of Application
Alosetron Hydrochloride is a serotonin SR-3 (5HT3-receptor) antagonist
Definition
ChEBI: The hydrochloride salt of alosetron.
Manufacturing Process
4-(Chloromethyl)-1-(triphenylmethyl)-1H-imidazole.
Thionyl chloride (0.829 g) was added over 1 min to a stirred suspension of 1-
(triphenylmethyl)-1H-imidazole-4-methanol (1.3 g) in a mixture of
dichloromethane (50 ml) and DMF (1.0 ml) at 23°C. The solution so obtained
was stirred for 15 min. and extracted with 8% sodium bicarbonate solution
(80 ml). The organic phase was washed with water (50 ml), dried and
evaporated to give an oil which solidified. The solid was slurried in hexane and
filtered to give the title compound (1.28 g), m.p. 139-141°C.
3,4-Dihydro-4-methylcyclopent[b]indol-1(2H)-one oxime.
3,4-Dihydro-4-methylcyclopent[b]indol-1(2H)-one (1.7 g) and hydroxylamine
hydrochloride (1.925 g) in pyridine were heated at 60°C for 18 h and cooled.
The reaction mixture was evaporated in vacuo to a residue to which was
added 8% sodium bicarbonate (150 ml). Extraction with ethyl acetate (300
ml) produced a suspension in the organic layer; this layer and associated solid
was separated from the aqueous layer. The aqueous layer was re-extracted
with ethyl acetate (250 ml). The combined organic extracts (and suspended
solid) were evaporated to a residue, boiled with a mixture of ethanol (150 ml)
and methanol (150 ml) and cooled to 50°C. The residue was adsorbed from
this solution on to FCC silica and applied to an FCC column. Elution with ethyl
acetate/3-10% methanol provided the title compound (1.69 g), m.p. 219-
224°C (decomp.).
2,3,4,5-Tetrahydro-5-methyl-1H-pyrido[4,3-b]indol-1-one.
3,4-Dihydro-4-methylcyclopent[b]indol-1(2H)-one oxime (1.53 g),
polyphosphoric acid (409 g) and dioxan (15 ml) were heated at 110-120°C for
2.2 h under nitrogen. The reaction mixture was cooled, and treated with 2 N
sodium carbonate solution (1 L). The suspension was extracted with ethyl
acetate (4x400 ml) and the combined extracts were dried. Evaporation gave a
solid (1.43 g) which was recrystallised from ethyl acetate/cyclohexane. This
solid was purified by FCC, eluting with dichloromethane:ethanol:ammonia
solution (200:10:1) to give a solid (1.26 g) which was recrystallised from
ethanol to provide the title compound (960 mg), m.p. 234-238°C.
2,3,4,5-Tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-1Hpyrido[
4,3-b]indol-1-one maleate.
A mixture of 2,3,4,5-tetrahydro-5-methyl-1H-pyrido[4,3-b]indol-1-one (0.6 g)
and 78% sodium hydride dispersion in mineral oil (0.109 g) in dry DMF (15
ml) was stirred under nitrogen at 50°C until hydrogen evolution ceased (ca.
1.5 h). The mixture was cooled to 40°C and a solution of 4-(chloromethyl)-5-
methyl-1-(triphenylmethyl)-1H-imidazole (1.12 g) in dry THF (15 ml) was
added. The reaction was then stirred at 40°C for 3 h, at 20°C for 16 h and a
further portion of 4-(chloromethyl)-5-methyl-1-(triphenylmethyl)-1H-imidazole
(1.12 g) in dry THF (15 ml) was added. The resulting mixture was heated at
40°C for 3 h, quenched with water (20 ml) and acetic acid (20 ml), and
heated at 100°C for 2 h. The mixture was then concentrated in vacuo to ca.
60 ml, diluted with 1 M hydrochloric acid (40 ml) and washed with ethyl
acetate (3x50 ml). The organic phase was discarded and the acidic aqueous
phase was basified (pH=9) with potassium carbonate and extracted with ethyl
acetate:ethanol (20:1; 3x100 ml). The extracts were combined, dried and
evaporated to give a brown gum (ca. 1 g). This gum was adsorbed onto silica
and purified by FCC eluting with dichloromethane:ethanol:ammonia solition
(100:8:1) to give a pale brown solid (0.8 g); m.p. 238-240°C (decomp.). This
solid was dissolved in a mixture of (hot ethanol and methanol (1:1; 100 ml)
and treated with an ethanolic solution of maleic acid (3.18 g). The resulting
solution was concentrated to ca. 20 ml and diluted with dry diethyl ether (ca.
8 ml) to precipitate the title compound (0.75 g) as an off-white solid melting
point 160-162°C. Hydrochloride may be prepared by treating the above solid
with an equivalent of an ethanolic solution of HCl.
brand name
Lotronex (GlaxoSmithKline).
Therapeutic Function
Antidiarrheal
Biochem/physiol Actions
Alosetron is a potent and highly selective antagonist of serotonin 5-HT3 receptors, nonselective cation channels found predominantly in the enteric nervous system of the gastrointestinal tract. These receptors are involved in the regulation of visceral pain, colonic transit and GI secretions that can contribute to the pathophysiology of irritable bowel syndrome (IBS). Alosetron is used clinically for treatment of women with severe diarrhea-predominant IBS.
Environmental Fate
The environmental fate and behavior of Lotronex is uncertain, as formal studies regarding its release into the environment are virtually nonexistent in the literature. Limited water solubility (61 mg ml-1) may lead to persistence in soils, and in sediment following aquatic release, although specific fates with regard to degradation, bioaccumulation, and transport are unknown.
Toxicity evaluation
A highly potent and selective 5-HT3 receptor agonist, Lotronex rapidly binds to cation channels that exist on enteric neurons in the human gastrointestinal tract in addition to other central and peripheral locations. Lotronex inhibits the activation of these channels, resulting in effects to the enteric nervous system whereas the activation of these channels regulates (promotes) colonic transit, visceral pain pathways, and gastrointestinal secretions – all of which are processes related to the pathophysiology of IBS. The binding of Lotronex to 5-HT3 receptors reduces the rate at which fecal matter moves through the large intestine and increases water absorption. The toxic effects of Lotronex are related to the relative activation/inactivation of the cation channels responsible for mediating these processes.
Properties of Alosetron hydrochloride
| Melting point: | 288-291°C |
| storage temp. | 2-8°C |
| solubility | H2O: ≥5mg/mL at warmed |
| form | powder |
| color | white to beige |
| CAS DataBase Reference | 122852-69-1(CAS DataBase Reference) |
Safety information for Alosetron hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Alosetron hydrochloride
Alosetron hydrochloride manufacturer
Aruvi Labs
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole hydrochloride](https://img.chemicalbook.in/CAS/GIF/20522-30-9.gif)
![2,3,4,5-Tetrahydro-5-methyl-1H-pyrido[4,3-b]indol-1-one](https://img.chemicalbook.in/CAS/GIF/122852-75-9.gif)
![1-METHYL-4,5,6,7-TETRAHYDRO-1H-PYRROLO[3,2-C]PYRIDINE](https://img.chemicalbook.in/CAS/GIF/569351-26-4.gif)
You may like
-
 122852-69-1 Alosetron hydrochloride 98%View Details
122852-69-1 Alosetron hydrochloride 98%View Details
122852-69-1 -
 Alosetron hydrochloride 98%View Details
Alosetron hydrochloride 98%View Details -
 Alosetron hydrochloride 98%View Details
Alosetron hydrochloride 98%View Details
122852-69-1 -
 Alosetron hydrochloride 98%View Details
Alosetron hydrochloride 98%View Details
122852-69-1 -
 Alosetron Hydrochloride CAS 122852-69-1View Details
Alosetron Hydrochloride CAS 122852-69-1View Details
122852-69-1 -
 Alosetron HCl 98% (HPLC) CAS 122852-69-1View Details
Alosetron HCl 98% (HPLC) CAS 122852-69-1View Details
122852-69-1 -
 Alosetron hydrochloride CAS 122852-69-1View Details
Alosetron hydrochloride CAS 122852-69-1View Details
122852-69-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
