ALOGLIPTIN(ALOGLIPTINE, ALOGLIPTINA)
- CAS NO.:850649-61-5
- Empirical Formula: C18H21N5O2
- Molecular Weight: 339.39
- MDL number: MFCD09833196
- EINECS: 821-899-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
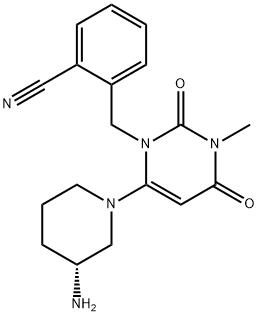
What is ALOGLIPTIN(ALOGLIPTINE, ALOGLIPTINA)?
Absorption
The pharmacokinetics of NESINA was also shown to be similar in healthy subjects and in patients with type 2 diabetes. When single, oral doses up to 800 mg in healthy subjects and type 2 diabetes patients are given, the peak plasma alogliptin concentration (median Tmax) occurred 1 to 2 hours after dosing. Accumulation of aloglipin is minimal. The absolute bioavailability of NESINA is approximately 100%. Food does not affect the absorption of alogliptin.
Toxicity
Common adverse reactions (reported in ≥4% of patients treated with alogliptin 25 mg and more frequently than in patients who received placebo) are: nasopharyngitis, headache, and upper respiratory tract infection.
Description
Alogliptin is a dipeptidyl-peptidase IV (DPP-4) inhibitor that was approved in Japan in 2010 for treatment of type 2 diabetes, a disease in which insulin resistance and β-cell dysfunction lead to hyperglycemia. As islet function is lost, the severity of insulin resistance increases. The introduction of DPP-4 inhibitors has brought a novel class of insulinotropic agents for the treatment options available to type 2 diabetic patients. The therapeutic potential of glucagon-like peptide 1 (GLP-1), an incretin peptide, for the treatment of type 2 diabetes was realized in the 1990s. The insulinotropic effects of GLP-1 depend closely on glucose concentrations providing the possibility of glucose normalization without the risk of hypoglycemia. GLP-1 has other non-insulinotropic physiological actions that are advantageous. It suppresses glucagon secretion from a cells and slows gastric emptying, which contributes to satiety and to a slower passage and reabsorption of carbohydrates. GLP-1 also contributes to satiety via a central mechanism as a neurotransmitter with effects on the hypothalamus.
Chemical properties
White Solid
Originator
Syrrx Inc. (now Takeda San Diego) (Japan)
The Uses of ALOGLIPTIN(ALOGLIPTINE, ALOGLIPTINA)
Alogliptin is an oral antihyperglycemic agent that is a selective inhibitor of the enzyme dipeptidyl peptidase-4 (DPP-4). Antidiabetic agent.
Background
Alogliptin is a selective, orally-bioavailable inhibitor of enzymatic activity of dipeptidyl peptidase-4 (DPP-4). Chemically, alogliptin is prepared as a benzoate salt and exists predominantly as the R-enantiomer (>99%). It undergoes little or no chiral conversion in vivo to the (S)-enantiomer. FDA approved January 25, 2013.
Indications
Indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Definition
ChEBI: A piperidine that is 3-methyl-2,4-dioxo-3,4-dihydropyrimidine carrying additional 2-cyanobenzyl and 3-aminopiperidin-1-yl groups at positions 1 and 2 respectively (the R-enantiomer). Used in the form of its benzoate salt for treatment of t pe 2 diabetes.
brand name
Nesina
Pharmacokinetics
Peak inhibition of DPP-4 occurs within 2-3 hours after a single-dose administration to healthy subjects. The peak inhibition of DPP-4 exceeded 93% across doses of 12.5 mg to 800 mg. Inhibition of DPP-4 remained above 80% at 24 hours for doses greater than or equal to 25 mg. Alogliptin also demonstrated decreases in postprandial glucagon while increasing postprandial active GLP-1 levels compared to placebo over an 8-hour period following a standardized meal. Alogliptin does not affect the QTc interval.
Clinical Use
Dipeptidyl peptidase 4 inhibitor:
Treatment of type 2 diabetes in combination with
other therapies
Metabolism
Alogliptin does not undergo extensive metabolism. Two minor metabolites that were detected are N-demethylated alogliptin (<1% of parent compound) and N-acetylated alogliptin (<6% of parent compound). The N-demethylated metabolite is active and an inhibitor of DPP-4. The N-acetylated metabolite is inactive. Cytochrome enzymes that are involved with the metabolism of alogliptin are CYP2D6 and CYP3A4 but the extent to which this occurs is minimal. Approximately 10-20% of the dose is hepatically metabolized by cytochrome enzymes.
Metabolism
Alogliptin does not undergo extensive metabolism. Two minor metabolites were detected following administration of an oral dose of [14C]-alogliptin, N-demethylated alogliptin, M-I (<1% of the parent compound), and N-acetylated alogliptin, M-II (<6% of the parent compound). M-I is an active metabolite and is a highly selective inhibitor of DPP-4 similar to alogliptin; M-II does not display any inhibitory activity towards DPP-4 or other DPP-related enzymes. In vitro data indicate that CYP2D6 and CYP3A4 contribute to the limited metabolism of alogliptin.
Properties of ALOGLIPTIN(ALOGLIPTINE, ALOGLIPTINA)
| Melting point: | 127 - 129°C |
| Boiling point: | 519.2±60.0 °C(Predicted) |
| Density | 1.342 |
| Flash point: | 267.8℃ |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.89±0.20(Predicted) |
| color | White to Off-White |
Safety information for ALOGLIPTIN(ALOGLIPTINE, ALOGLIPTINA)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ALOGLIPTIN(ALOGLIPTINE, ALOGLIPTINA)
ALOGLIPTIN(ALOGLIPTINE, ALOGLIPTINA) manufacturer
ALS India Life Sciences Pvt. Ltd
SRINI PHARMACEUTICALS PVT LTD
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Alogliptin 98%View Details
Alogliptin 98%View Details -
 850649-61-5 98%View Details
850649-61-5 98%View Details
850649-61-5 -
 Alogliptin 98%View Details
Alogliptin 98%View Details
850649-61-5 -
 Alogliptin 850649-61-5 98%View Details
Alogliptin 850649-61-5 98%View Details
850649-61-5 -
 850649-61-5 Alogliptin 99%View Details
850649-61-5 Alogliptin 99%View Details
850649-61-5 -
 Alogliptin 98%View Details
Alogliptin 98%View Details -
 Alogliptin 99% (HPLC) CAS 850649-61-5View Details
Alogliptin 99% (HPLC) CAS 850649-61-5View Details
850649-61-5 -
 Alogliptin 95.00%View Details
Alogliptin 95.00%View Details
850649-61-5
