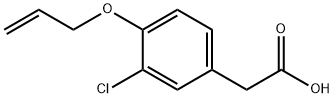
ALCLOFENAC
- CAS NO.:22131-79-9
- Empirical Formula: C11H11ClO3
- Molecular Weight: 226.66
- MDL number: MFCD00071612
- EINECS: 244-795-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is ALCLOFENAC?
Absorption
The absorption of alclofenac from the gastrointestinal tract is irregular. After oral or rectal administration maximum plasma concentrations are reached within 1-4 hours.
Toxicity
The lethal dose orally: 1100 (mice), 1050 (rats) mg / kg ; under the skin: 600 (mice), 630 (rats) mg / kg intraperitoneally: 550 (mice), 530 (rats) mg / kg
Chemical properties
Off-White to Pale Yellow Solid
Originator
Allopydin ,Chugai ,Japan ,1976
The Uses of ALCLOFENAC
Analgesic; antipyretic; anti-inflammatory.
Definition
ChEBI: An aromatic ether in which the ether oxygen links an allyl group to the 4-position of (3-chlorophenyl)acetic acid.A non-steroidal anti-inflammatory drug, it was withdrawn from the UK market in 1979 due to concerns with its association with vasculitis and r sh.
Indications
Alclofenac is indicated in rheumatology, in particular for the treatment of rheumatoid arthritis, ankylosing spondylitis and, as an analgesic, in painful arthritic pathologies.
What are the applications of Application
Alclofenac is An anti-inflammatory agent
Background
Alclofenac is a non-steroidal anti-inflammatory drug. It was withdrawn from the market in the United Kingdom in 1979.
Manufacturing Process
103.7 grams of 3-chloro-4-allyloxyphenylacetonitrile in 500 cc of ethanol, 100 grams of potassium hydroxide and 100 cc of water are refluxed for 4 hours. Maximum of alcohol is evaporated, the residue is diluted with water and ice, and acidified with 20% HCl. The solid is filtered and washed with petroleum ether. 91.5 grams of acid are obtained (Yield: 81%) which is recrystallized from aqueous methanol; MP 92-93°C.
brand name
Mervan (Continental Pharma, Belgium);Allopydinac;Alopidin;Alopydin;Argan;Darkeyfenac;Desinflam;Medifenac;Mevan;Mirvan a;Prinalgin;Vanadian;W-7320;Zubirol.
Therapeutic Function
Antiinflammatory
World Health Organization (WHO)
Alclofenac, a phenylacetic acid derivative with analgesic, antipyretic and antiinflammatory activity, was introduced in 1972 for the treatment of rheumatic disorders. In the late 1970s its use was associated with a high incidence of adverse effects, mainly skin rashes, and a urinary metabolite was reported to have mutagenic activity (positive Ames test). This resulted in the withdrawal of the drug, in some cases voluntarily, from several countries. In others registration has been refused. The reported mutagenic potential has been questioned by some investigators and the drug remains on the market in at least three countries with highly evolved regulatory authorities.
Metabolism
the main metabolic product is alclofenac itself and alclofenac glucuronide
Dosage
Alclofenac when used at 3 g/d in the treatment of rheumatoid arthritis is as effective as 75 mg/d of indomethacin with fewer adverse effects. In the treatment of osteoarthritis 1500 mg/d is as effective and is better tolerated than 300 mg/d of phenylbutazone. Alclofenac has been withdrawn from the market in the United Kingdom because of skin rash and vasculitis (blood vessel inflammation).
Properties of ALCLOFENAC
| Melting point: | 90-91°C |
| Boiling point: | 324.6°C (rough estimate) |
| Density | 1.2401 (rough estimate) |
| refractive index | 1.4530 (estimate) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | pKa 10.3 (Uncertain) |
| form | Solid |
| color | Off-White to Pale Yellow |
| Water Solubility | 13.1mg/L(25 ºC) |
Safety information for ALCLOFENAC
Computed Descriptors for ALCLOFENAC
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
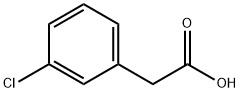
 (4-(ALLYLOXY)-3-CHLOROPHENYL)ACETIC ACID CAS 22131-79-9View Details
(4-(ALLYLOXY)-3-CHLOROPHENYL)ACETIC ACID CAS 22131-79-9View Details
22131-79-9 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1