Alanosine
- CAS NO.:5854-93-3
- Empirical Formula: C3H7N3O4
- Molecular Weight: 149.11
- MDL number: MFCD00866314
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
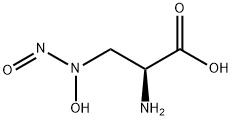
What is Alanosine?
Chemical properties
Crystalline Powder
Originator
Alanosine ,Triangle Pharmaceuticals
The Uses of Alanosine
L-Alanosine is an antitumor antibiotic substance from the fermentation of Streptomyces alanosinicus. It is the first natural product found to have a N-nitrosohydroxylamino group on an aliphatic chain. It is also an experimental insect reproduction inhibitor.
The Uses of Alanosine
Antibiotic substance from the fermentation of Streptomyces alanosinicus. The first natural product found to have a N-nitrosohydroxylamino group on an aliphatic chain. An experimental insect reproduction inhibitor
What are the applications of Application
L-Alanosine is a natural product found to have a N-nitrosohydroxylamino group on an aliphatic chain
Manufacturing Process
Alanosine is an antibiotic isolated from the fermentation broth of
Streptomyces alanosinicus n. sp.
Oat meal agar slants seeded with Streptomyces alanosinicus n. sp. ATCC
15710 were incubated at 20°C for 7 to 10 days and then used to inoculate
100 ml of a peptone-agar-glucose-yeast extract medium contained in 500 ml
Erlenmeyer flask. The composition of this fermination medium is:
Meat extract 5.0 g./liter
Peptone 5.0 g./liter
Yeast extract 5.0 g./liter
Enzymatic casein hydrolysate 3.0 g./liter
Cerelose 2.0 g./liter
NaCl 1.5 g./liter
The medium is adjusted to pH 7.2 prior to sterilization for 20 minutes at
121°C and 15 Ibs steam pressure. The germination flasks are incubated at
28°C for 48 hours on rotary shaker having a 2 inch throw and making 240
rpm. A 3% transfer is made from the germination flask to 500 ml Erlenmeyer
fermentation flaks containing 100 ml of medium TVF/5 having the following
composition:
Glucose 50.0 g/L
Dried whale meat (Pascor) 10.0 g/L
CaCO4 5.0 g/L
(NH4)2SO4 1.0 g/L
MgSO4·7H2O 1.0 g/L
CuSO4 sol. 0.5% 1 ml
FeSO4 sol. 0.1% 1 ml
ZnSO4 sol. 0.2% 1 ml
MnSO4 sol. 0.8% 1 ml
The medium is adjusted to pH 7.0 prior to sterilization for 20 minutes at
121°C. The fermentation flasks are incubated and agitated under similar
conditions as the germination flasks. After 72 hours the mycelium was
separated by centrifugation and the untreated broth assayed by the streak
dilution method. 30 liters of broth are centrifuged and 6% of Darco G-60
charcoal is added to the clear solution, which is stirred for 30 minutes; then
the charcoal is filtered off to give an almost colorless solution which is
concentrated in vacuum at 45-50°C to 1.5 liters. The concentrated solution is
poured under stirring into 5 liters of methanol, the formed precipitate is filtered, washed with much acetone and dried in vacuum. Yield 230 g of crude
product assaying about 10%.
Therapeutic Function
Antineoplastic
Enzyme inhibitor
This antibiotic and aspartate/asparagine analogue (FW = 149.11 g/mol; CAS 5854-93-3), also named L-2-amino-3-(N-hydroxy-N-nitrosoamino) propionic acid, isolated from fermentation broths of Streptomyces alanosinicus, inhibits adenylosuccinate synthetase, disrupting de novo synthesis of AMP in both malignant and normal cells. That alanosine inhibits the adenylosuccinate synthetase reaction, the first step in the conversion of IMP to AMP, is evidenced by the accumulation of IMP, but not adenylosuccinate, in treated cells. L-Alanosine’s action is potentiated in methylthioadenosine phosphorylase (MTAP) deficiency. Clinical use is limited by its toxicity. At a concentration as low as 2.7 μM, L-alanosine completely inhibits the incorporation of hypoxanthine into adenosine triphosphate by cultured Novikoff rat hepatoma cells. A related agent, L-alanosyl-5-aminoimidazole-4-carboxylic acid ribonucleotide (or alanosyl-AICOR) has been synthesized enzymatically using 4-(N-succino)- 5-aminoimidazole-4-carboxamide ribonucleotide (or SAICAR) synthetase in conjunction with 5-aminoimidazole-4-carboxylic acid ribonucleotide and alanosine. Alanosyl-AICOR is not a substrate of adenylosuccinate lyase from rat skeletal muscle, but it was an apparent competitive inhibitor in both reactions catalyzed by the enzyme.
Properties of Alanosine
| Melting point: | 1900C (dec.) |
| Boiling point: | 270.14°C (rough estimate) |
| alpha | D +8°, -46°, -37.8° (in 1N HCl, 0.1N NaOH, water) |
| Density | 1.6720 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| storage temp. | -20°C Freezer |
| solubility | Aqueous Acid, Aqueous Base |
| form | Solid |
| pka | 4.8(at 25℃) |
| color | Off-White to Pale Beige |
Safety information for Alanosine
Computed Descriptors for Alanosine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4