Afloqualone
- CAS NO.:56287-74-2
- Empirical Formula: C16H14FN3O
- Molecular Weight: 283.3
- MDL number: MFCD00867693
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-07-07 16:22:03
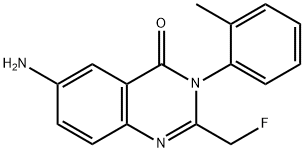
What is Afloqualone?
Description
Afloqualone is a centrally acting muscle relaxant useful in the management of various spastic conditions, including cerebral palsy, cervical spondylosis, and multiple sclerosis. It is closely related to the hypnotidsedative methaqualone.
Description
Afloqualone (Item No. 21834) is an analytical reference standard categorized as a quinazolinone. This product is intended for research and forensic applications.
Originator
Tanabe (Japan)
The Uses of Afloqualone
Afloqualone acts as a muscle relaxant in humans and may be used in the treatment of muscle spasms affecting rheumatoid arthritis.
Definition
ChEBI: Afloqualone is an organic molecular entity.
Manufacturing Process
14.4 g (0.053 mol) of N-(2-amino-5-nitrobenzoyl)-o-toluidine and 6.3 g (0.08
mol) of pyridine are dissolved in 300 ml of tetrahydrofuran. 12.2 g (0.126
mol) of fluoroacetyl chloride are added to the solution for 10 minutes under
ice-cooling. The solution is stirred at the same temperature for 30 minutes
and then at room temperature for 2.5 hours. The reaction solution is allowed
to stand at room temperature overnight. The crystalline precipitate is collected
by filtration, washed with water and then dried. 16.4 g of N-(2-
fluoroacetamido-5-nitrobenzoyl)-o-toluidine are obtained. Yield: 93.7%; MP
238-239°C.
16.5 g (0.05 mol) of N-(2-fluoroacetamido-5-nitrobenzoyl)-o-toluidine and
25.5 g (0.25 mol) of acetic acid anhydride are dissolved in 250 ml of glacial
acetic acid. The solution is refluxed for 2 hours under heating. Then, the
reaction solution is evaporated to remove solvent. The residue thus obtained
is poured into ice-water, and the aqueous mixture is adjusted to pH 9 with
potassium carbonate. The crystalline precipitate is collected by filtration. 15.5
g of 2-fluoromethyl-3-(o-tolyl)-6-nitro-4(3H)-quinazolinone are obtained.
Yield: 98.7%; MP 155-158°C (recrystallized from ethanol).
A mixture of 2.0 g (0.064 mol) of 2-fluoromethyl-3-(o-tolyl)-6-nitro-4(3H)-
quinazolinone, 0.2 g of 5% palladium-carbon and 100 ml of acetic acid is
shaken for 30 minutes in hydrogen gas. The initial pressure of hydrogen gas is
adjusted to 46 lb and the mixture is heated with an infrared lamp during the
reaction. After 30 minutes of this reaction, the pressure of hydrogen gas
decreases to 6 lb. After the mixture is cooled, the mixture is filtered to
remove the catalyst. The filtrate is evaporated to remove acetic acid, and the
residue is dissolved in chloroform. The chloroform solution is washed with 5%
aqueous sodium hydroxide and water, successively. Then, the solution is dried
and evaporated to remove solvent. The oily residue thus obtained is dissolved
in 2 ml of chloroform, and the chloroform solution is passed through a column
of 200 g of silica gel. The silica gel column is eluted with ethyl acetatebenzene
(1:1). Then, the eluate is evaporated to remove solvent. The crude
crystal obtained is washed with isopropyl ether and recrystallized from isopropanol. 0.95 g of 2-fluoromethyl-3-(o-tolyl)-6-amino-4(3H)-quinazolinone
is obtained. Yield: 52.5%; MP 195-196°C.
brand name
AROFUTO
Therapeutic Function
Muscle relaxant
Properties of Afloqualone
| Melting point: | 195-196°C |
| Boiling point: | 492.5±55.0 °C(Predicted) |
| Density | 1.2529 (estimate) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 2.73±0.70(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C16H14FN3O/c1-10-4-2-3-5-14(10)20-15(9-17)19-13-7-6-11(18)8-12(13)16(20)21/h2-8H,9,18H2,1H3 |
| CAS DataBase Reference | 56287-74-2(CAS DataBase Reference) |
Safety information for Afloqualone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Afloqualone
| InChIKey | VDOSWXIDETXFET-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=C(N)C=C2)C(=O)N(C2=CC=CC=C2C)C=1CF |
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 Afloqualone 99% (HPLC) CAS 56287-74-2View Details
Afloqualone 99% (HPLC) CAS 56287-74-2View Details
56287-74-2 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4