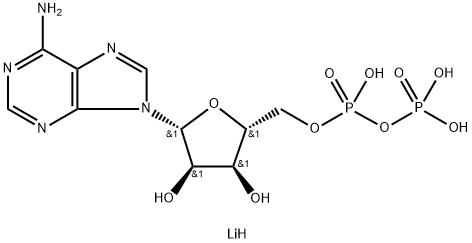
Adenosine triphosphate
- CAS NO.:56-65-5
- Empirical Formula: C10H16N5O13P3
- Molecular Weight: 507.18
- MDL number: MFCD00065467
- EINECS: 200-283-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-15 19:19:13
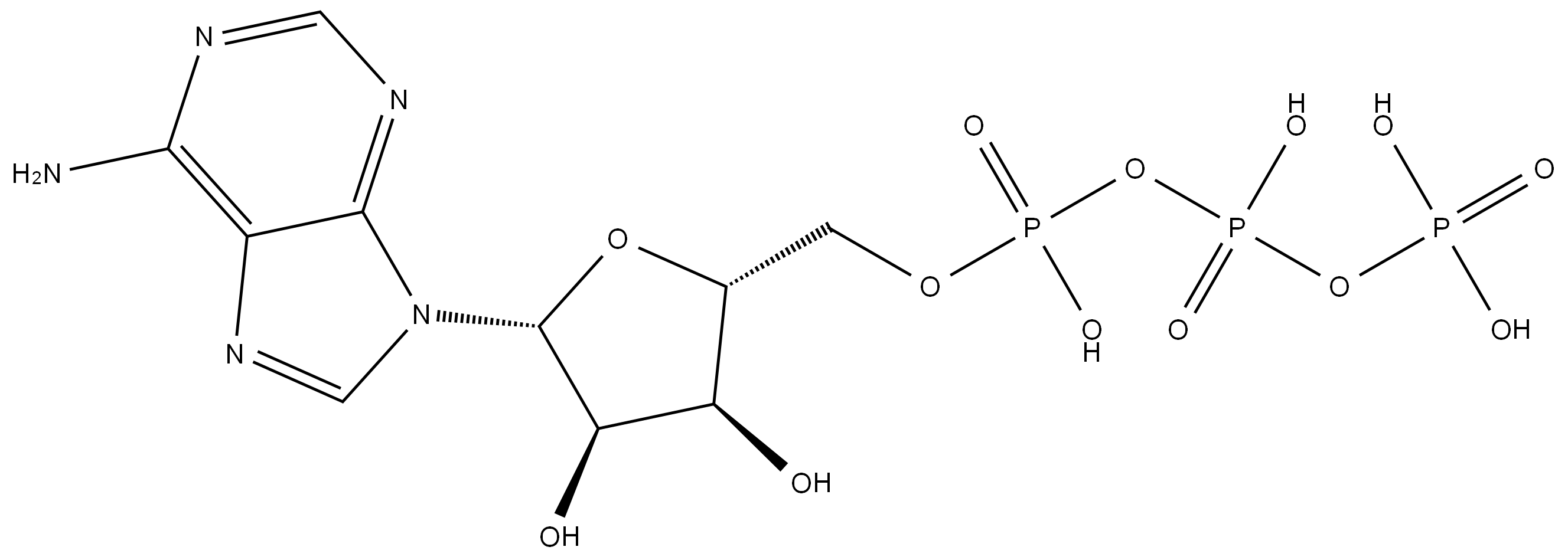
What is Adenosine triphosphate?
Description
Adenosine triphosphate, also known as ATP, is a molecule that carries energy within cells. It is one of the most important biological compounds because of its role in supplying energy for life. ATP is the universal energy carrier used by all organisms to supply energy for biological functions. It is often referred to as the energy currency of cells.
ATP also functions as a neurotransmitter that is stored and secreted with other neurotransmitters from the pancreas. ATP is a nucleotide consisting of the nucleoside adenosine with three attached phosphate groups (see Adenine). Like other nucleotides, ATP consists of three parts: a sugar, an amine base, and a phosphate group. The central part of the molecule in ATP is the sugar ribose. The amine base adenine is attached to the ribose, forming adenosine. Opposite the adenine on the ribose is attached a chain of three phosphate groups.
Description
Adenosine 5′-triphosphate, abbreviated ATP and usually expressed without the 5′-, is an important “energy molecule” found in all life forms. Specifically, it is a coenzyme that works with enzymes such as ATP triphosphatase to transfer energy to cells by releasing its phosphate groups. The molecule consists of three components: an adenine bicyclic system, a furanose ring, and a triphosphate chain.
Two research groups reported the discovery of ATP in 1929. Cyrus H. Fiske and Yellapragada Subbarow at Harvard Medical School (Boston) isolated it from mammalian muscle and liver. Likewise, Karl Lohmann at the Kaiser Wilhelm Institutes (Berlin and Heidelberg) identified it in muscle tissues.
ATP isolation from other sources followed over the next 15 years. Koscak Maruyama at Chiba University (Japan) wrote a comprehensive review of the discovery and structure elucidation of ATP in 1987.
ATP is biosynthesized in several ways, as described by Biology Dictionary:
Photophosphorylation is a method specific to plants and cyanobacteria. It is the creation of ATP from ADP using energy from sunlight, and occurs during photosynthesis. ATP is also formed from the process of cellular respiration in the mitochondria of a cell. This can be through aerobic respiration, which requires oxygen, or anaerobic respiration, which does not. Aerobic respiration produces ATP (along with carbon dioxide and water) from glucose and oxygen. Anaerobic respiration uses chemicals other than oxygen, and this process is primarily used by archaea and bacteria that live in anaerobic environments. Fermentation is another way of producing ATP that does not require oxygen; it is different from anaerobic respiration because it does not use an electron transport chain. Yeast and bacteria are examples of organisms that use fermentation to generate ATP.
ATP synthesized in mitochondria is the primary energy source for important biological functions, such as muscle contraction, nerve impulse transmission, and protein synthesis. According to Susanna T?rnroth-Horsefield and Richard Neutze at the University of Gothenburg (G?teborg, Sweden), “On any given day you turn over your body weight equivalent in ATP, the principal energy currency of the cell.”
Chemical properties
The structure of Adenosine triphosphate has an ordered carbon compound as a backbone, but the part that is really critical is the phosphorous part - the triphosphate. Three phosphorous groups are connected by oxygens to each other, and there are also side oxygens connected to the phosphorous atoms. Under the normal conditions in the body, each of these oxygens has a negative charge, and therefore repel each other. These bunched up negative charges want to escape - to get away from each other, so there is a lot of potential energy here.
If you remove just one of these phosphate groups from the end, so that there are just two phosphate groups, the molecule is much happier. This conversion from ATP to ADP is an extremely crucial reaction for the supplying of energy for life processes. Just the cutting of one bond with the accompanying rearrangement is sufficient to liberate about 7.3 kilocalories per mole = 30.6 kJ/mol. This is about the same as the energy in a single peanut.
hyperphysics.phy-astr.gsu.edu
Originator
Atepodin ,Medix ,Spain
History
ATP was first isolated by the German chemist Karl Lohmann (1898–1978) from muscle tissue extracts in 1929. Alexander Todd’s (1907–1997) research helped to clarify ATP’s structure, and it was first synthesized by Todd in 1948.
The Uses of Adenosine triphosphate
Adenosine triphosphate (ATP) plays a critical role in the transport of macromolecules such as proteins and lipids into and out of the cell. The hydrolysis of ATP provides the required energy for active transport mechanisms to carry such molecules across a concentration gradient.
Definition
Adenosine triphosphate (ATP) is the source of energy for use and storage at the cellular level. It is an adenosine 5'-phosphate in which the 5'-phosphate is a triphosphate group. It is involved in the transportation of chemical energy during metabolic pathways.
Synthesis
ATP is synthesized in organisms by several related mechanisms. Oxidative phosphorylation is the main process that aerobic organisms use to produce ATP. Oxidative phosphorylation produces ATP from ADP and inorganic phosphate (Pi) from the oxidation of nicotinamide adenine dinucleotide (NADH) by molecular oxygen in the cell’s mitochondria.
Glycolysis is another process that generates ATP. Glycolysis converts glucose into pyruvate and in the process also forms NADH and ATP. The process can be represented as: Glucose + 2ADP + 2NAD+ + 2Pi → 2 pyruvate + 2ATP + 2NADH + 2H+. In this reaction Pi represents free inorganic phosphate. The rate of glycolysis in the body is inversely related to the amount of available ATP. Pyruvate produced by glycolysis can enter the Krebs cycle, producing more ATP.
Manufacturing Process
With a solution of 0.29 part by weight of well dried 1,3- dicyclohexylguanidinium adenosine 5'-phosphoramidate in 5 parts by volume of ortho-chlorophenol is admixed a solution of 0.95 part by weight of bistriethylammonium pyrophosphate in a mixed solvent composed of 1 part by volume of ortho-chlorophenol and 2 parts by volume of acetonitrile. The mixture is left standing at 20°C for 2 days. Then 30 parts by volume of water is added to the mixture. After washing with three 15 parts by weight volumeportions of diethyl ether, the aqueous layer is separated, and the remaining diethyl ether in the aqueous layer is removed under reduced pressure. Five parts by weight of activated charcoal is added to the aqueous layer and the mixture is stirred for 30 minutes. The activated charcoal is filtered and further 1 part by weight of activated charcoal is added to the filtrate. After 20 minutes agitation, the activated charcoal is taken out by filtration. The combined activated charcoal is washed with a little water, and eluted twice with respective 300 and 200 parts by volume-portions of 50% (volume) ethanol containing 2% (volume) of concentrated aqueous ammonia. The eluate is concentrated to 40 parts by volume, then is passed through a column packed with 20 parts by volume of a strongly basic anion exchange resin in bead form (chloric type) (polystyrene trimethylbenzyl ammonium type resin sold under the name of Dowex-1 from Dow Chemical Company, Mich. USA). Then, the column is washed with 750 parts by volume of an acid aqueous saline solution containing 0.01 normal hydrochloric acid and 0.02 normal sodium chloride and then eluted with 600 parts by volume of an acid aqueous saline solution composed of 0.01 normal hydrochloric acid and 0.2 normal sodium chloride. After neutralizing with a diluted sodium hydroxide solution, the eluate is treated with activated charcoal to adsorb ATP as its sodium salt. The separated activated charcoal is washed with water and eluted with 60% (volume) ethanol containing 2% (volume) of concentrated aqueous ammonia. The eluate is concentrated to 0.5 part by volume, then 5 parts by volume of ethanol is added. The precipitate thus deposited is centrifuged and dried at low temperature to obtain 0.155 part by weight of tetra-sodium salt of ATP containing 4 mols of water of crystallization as a colorless crystalline powder. The yield is 47% relative to the theoretical.
Therapeutic Function
Coenzyme, Vasodilator
Agricultural Uses
Adenosine triphosphate (ATP) is a nucleotide of fundamental importance as a carrier of chemical energy in all living organisms. The most important function of phosphorus in a plant system is to store and transfer energy. During biochemical processes, ATP gets synthesized to store releasable energy with the breakdown of ATP to adenosine triphosphate (ADP) and to phosphate ion by dephosphorylation. Here, ADP and ATP act as energy currency within the plant.
Safety Profile
Poison by intraperitoneal route.Human mutation data reported. When heated todecomposition it emits toxic fumes of POx and NOx.
Purification Methods
ATP is purified by precipitating it as the barium salt on adding excess barium acetate solution to a 5% solution of ATP in water. The precipitate is filtered off, washed with distilled water, dissolved in 0.2M HNO3 and again precipitated with barium acetate. The precipitate, after several washings with distilled water, is redissolved in 0.2M HNO3, and slightly more than an equivalent of 0.2M H2SO4 is added to precipitate all the barium as BaSO4 which is filtered off. The ATP is then precipitated by addition of a large excess of 95% ethanol. It is filtered off, washed several times with 100% EtOH and finally with dry diethyl ether. It is dried in vacuo. [Kashiwagi & Rabinovitch J Phys Chem 59 498 1955, Beilstein 26 III/IV 3654.]
Properties of Adenosine triphosphate
| Melting point: | 144°C (rough estimate) |
| Boiling point: | 951.4±75.0 °C(Predicted) |
| alpha | D22 -26.7° (c = 3.095) |
| Density | 1.0 g/mL at 20 °C |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | ≈1 kg/l |
| form | lyophilized powder |
| appearance | white powder |
| pka | pK2: 4.00(-1);pK3: 6.48(-2) (25°C) |
| color | White to off-white |
| Water Solubility | Water : ≥ 100 mg/mL (197.17 mM) |
| CAS DataBase Reference | 56-65-5(CAS DataBase Reference) |
| EPA Substance Registry System | Adenosine triphosphate (56-65-5) |
Safety information for Adenosine triphosphate
Computed Descriptors for Adenosine triphosphate
| InChIKey | ZKHQWZAMYRWXGA-KQYNXXCUSA-N |
| SMILES | C(OP(=O)(O)OP(O)(=O)OP(O)(O)=O)[C@H]1O[C@@H](N2C3C(=C(N=CN=3)N)N=C2)[C@H](O)[C@@H]1O |
Abamectin manufacturer
Innovassynth Technologies (I) Ltd.
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 Adenosine-5'-triphosphoric acid 98%View Details
Adenosine-5'-triphosphoric acid 98%View Details
56-65-5 -
 Adenosine triphosphate 98% (HPLC) CAS 56-65-5View Details
Adenosine triphosphate 98% (HPLC) CAS 56-65-5View Details
56-65-5 -
 Adenosine triphosphate 95% CAS 56-65-5View Details
Adenosine triphosphate 95% CAS 56-65-5View Details
56-65-5 -
 Adenosine 5′-triphosphate, immobilized on Agarose 4B CASView Details
Adenosine 5′-triphosphate, immobilized on Agarose 4B CASView Details -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4