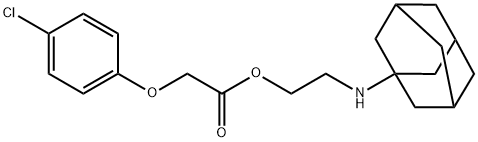
adafenoxate
- CAS NO.:82168-26-1
- Empirical Formula: C20H26ClNO3
- Molecular Weight: 363.88
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
What is adafenoxate?
Originator
Adafenoxate ,Laboratorios Wassermann
Manufacturing Process
Preparation of the p-chlorophenoxyacetate of 1-aminoadamantine-2-ethanol,
starting from a p-chlorophenoxyacetic acid halide:
22 g (0.11 mol) of 1-aminoadamantine-2-ethanol dissolved in 250 ml of
benzene are poured into a 500 ml flask fitted with a mechanical stirrer, using
a decanting funnel. 23 g (0.11 m) of p-chlorophenoxyacetyl chloride are
added in drops while stirring, the mixture then being stirred for 30 minutes.
120 ml of a 10% solution of sodium carbonate is then added, and the
resulting mixture is stirred for 10 minutes. The organic phase is decanted, and
the benzene is then removed by distillation. The residue is crystallized with
petroleum ether. This yields 37 g (93%) of a white solid.
Preparation of the p-chlorophenoxyacetate of 1-aminoadamantine-2-ethanol,
starting from p-chlorophenoxyacetic acid:
In a 1 liter flask, provided with a Dean-Stark separator tube and reflux
refrigerant, a mixture of 20.5 g (0.011 m) of p-chlorophenoxyacetic acid, 22 g
(0.11 m) of 1-aminoadamantine-2-ethanol, 98 g of conc. sulfuric acid and 700
ml of toluene is heated to boiling point over a period of 24 hours. At the end of this period, the mixture is treated with an aqueous solution of 5% sodium
carbonate to an alkali pH, and is then washed with water. The mixture is then
dried on anhydrous sodium sulphate, and the toluene is removed by
distillation at reduced pressure. The crude product so obtained is crystallized
with petroleum ether. The yield is 35.2 g (88%) of a white solid.
Preparation of the chlorhydrate of p-chlorophenoxyacetate of 1-
aminoadamantine-2-ethanol:
A solution of 60 g (0.16 m) of p-chlorophenoxyacetate of 1-aminoadamantine-
2-ethanol in 300 ml of ether is subjected to the passage of HCl gas until the
precipitation of a solid product is completed. It is left to cool in a refrigerator
over a period of 6 hours and it is then filtered. The resulting solid is
recrystallized with a mixture of ether and methanol. 61 g (92%) of the
product are obtained.
Therapeutic Function
Nootropic, Psychostimulant
Properties of adafenoxate
| Boiling point: | 489.2±30.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| pka | 8.85±0.20(Predicted) |
Safety information for adafenoxate
Computed Descriptors for adafenoxate
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8