Acetyl-trans-resveratrol
Synonym(s):trans-3,5,4ʹ-Triacetylstilbene, 3,5,4ʹ-Tri-O-acetylresveratrol;Resveratrol, Triacetyl - CAS 42206-94-0 - Calbiochem
- CAS NO.:42206-94-0
- Empirical Formula: C20H18O6
- Molecular Weight: 354.35
- MDL number: MFCD01546481
- EINECS: 695-560-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
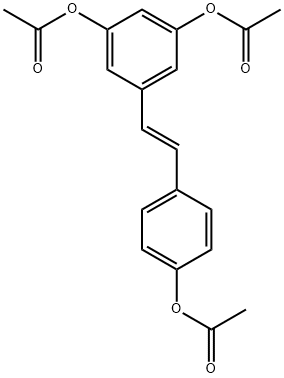
What is Acetyl-trans-resveratrol?
Description
Triacetyl resveratrol (CAS 42206-94-0) is a resveratrol prodrug. One method for increasing the half-life of resveratrol?in vivo?is acetylation of the phenolic OH groups. Deacetylation takes place?in vivo?or in intact cells via the action of intracellular esterases releasing active resveratrol.
Chemical properties
White crystal or white crystal
The Uses of Acetyl-trans-resveratrol
trans-Resveratrol Triacetate is the acetylated analogue of Resveratrol (R150000) that is a natural antioxidant and a potential radioprotective agent. trans-Resveratrol Triacetate induces p53 activity and inhibits proliferation in breast and prostate tumor cell lines.
The Uses of Acetyl-trans-resveratrol
trans-Resveratrol Triacetate is the acetylated analogue of Resveratrol (R150000) that is a natural antioxidant and a potential radioprotective agent. trans-Resveratrol Triacetate induces p53 activity and inhibits proliferation in breast and prostate tumor cell lines.
What are the applications of Application
Triacetylresveratrol is a resveratrol precursor and SIRT activator
Definition
ChEBI: Acetic acid [4-[2-(3,5-diacetyloxyphenyl)ethenyl]phenyl] ester is a stilbenoid.
General Description
A cell-permeable triacetate resveratrol prodrug that is easily converted to resveratrol by esterase activity and exhibits similar bioactivity as resveratrol in cell cultures. Cremophor EL is reported to protect the prodrug from fast hydrolysis and resveratrol conversion in mouse serum at 37°C (100% conversion within 20 sec vs. >300 min or T1/2 of 48 min delivered with DMSO or 1:1:8 (v/v/v) EtOH/Cremophor EL/H2O, respectively; [prodrug]t=0 = 100 μM), although the active drug resveratrol is stable for more than 27 h under the same condition without Cremophor EL. When dosed at 10 mg/kg and delivered with 1:1:8 (v/v/v) EtOH/Cremophor EL/H2O intraperitoneally before, but not after, exposure to lethal γ-irradiation (9.75 Gy), the prodrug, but not resveratrol, is shown to greatly prevent irradiation-induced death in mice in vivo (survival rate = 77.4% with 10 mg/kg prodrug vs. ≤28.3% with 10 mg/kg resveratrol, vehicle alone, or no pretreatment at all).
Biochem/physiol Actions
Triacetyl resveratrol displays superior bioavailability to the parent compound, resveratrol. The compound induces p53 activity and inhibits proliferation in breast and prostate tumor cell lines.
Mechanism of action
After acetylated resveratrol enters the body, it is converted into resveratrol in the body to exert its biological activity. Acetylated Resveratrol Compared to Regular Resveratrol:
1) The stability is enhanced. Since the three active phenolic hydroxyl groups are acetylated, the stability of acetylated resveratrol is significantly improved;
2) The bioavailability is improved, due to prolonging the residence time in the body and increasing the half-life, the bioavailability is better. Acetylated resveratrol can be widely used in food, beverage and health care products.
in vitro
Triacetylresveratrol significantly down-regulates anti-apoptotic Bcl-2 family protein Mcl-1 and up-regulates pro-apoptotic Bcl-2 family proteins Bim and Puma. Triacetylresveratrol inhibits cell viability, and induces apoptosis of pancreatic cancer cells in a concentration and incubation time-dependent manner.
References
1) Hsieh?et al.?(2011),?Control of prostate cell growth, DNA damage and repair and gene expression by resveratrol analogues, in vitro; Carcinogenesis,?32?93 2) Hsieh?et al.?(2011),?Regulation of p53 and cell proliferation by resveratrol and it’s derivatives in breast cancer cells: an in silico and biochemical approach to targeting integrin αvβ3; Int. J. Cancer,?129?2732
Properties of Acetyl-trans-resveratrol
| Melting point: | 117.0 to 121.0 °C |
| Boiling point: | 504.8±50.0 °C(Predicted) |
| Density | 1.241±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: ≥18mg/mL |
| form | White powder |
| color | white to tan |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
Safety information for Acetyl-trans-resveratrol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acetyl-trans-resveratrol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Triacetylresveratrol CAS 42206-94-0View Details
Triacetylresveratrol CAS 42206-94-0View Details
42206-94-0 -
 Acetyl-trans-resveratrol 98% CAS 42206-94-0View Details
Acetyl-trans-resveratrol 98% CAS 42206-94-0View Details
42206-94-0 -
 Resveratrol, Triacetyl CAS 42206-94-0View Details
Resveratrol, Triacetyl CAS 42206-94-0View Details
42206-94-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
