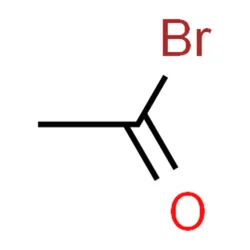
Acetyl bromide
Synonym(s):Acetic acid bromide, Acetoxy bromide;Acetyl bromide
- CAS NO.:506-96-7
- Empirical Formula: C2H3BrO
- Molecular Weight: 122.95
- MDL number: MFCD00000114
- EINECS: 208-061-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
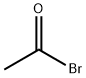
What is Acetyl bromide?
Description
Acetyl bromide is a colourless fuming liquid with pungent odour, is combustible, and turns yellow on exposure to air. It is used as an acetylating agent in the synthesis of fine chemicals, agrochemicals, and pharmaceuticals. It is also used as an intermediate for dyes. Acetylation, a case of acylation, is an organic synthesis process whereby the acetyl group is incorporated into a molecule by substitution for protecting –OH groups.
Chemical properties
Acetyl bromide is a colorless, fuming liquid that turns yellow on contact with air. It has a sharp, unpleasant odor. Soluble in benzene, ether and methyl chloride. Reacts violently with water or alcohol.
The Uses of Acetyl bromide
Acetyl Bromide is used as an acetylating agent in the synthesis of fine chemicals, agrochemicals and pharmaceuticals. It is also used as an intermediate for dyes. Further, it reacts with alcohols and amines to produce acetate esters and acetamides. In addition to this, it is involved in the synthetic process of the anti-HIV agent.
What are the applications of Application
Acetyl bromide can be used:
In the quantification of lignin in cell wall residues of Arabidopsis.
In the total synthesis of cinchona alkaloids named quinidine and quinine.
To prepare 9,10-diarylanthracenes and triarylmethanes by reacting with arenes and various aromatic aldehydes using ZnBr2/SiO2.
To prepare glycosyl bromides from free sugars or their peracetates.
Preparation
The preparation of acetyl bromide from acetic acid and bromine in the presence of red and yellow phosphorus.
Reaction: Acetic anhydride was brominated with bromine at 80°C, and then the bromine was added between 95°C and 125°C. About 2.5h. After the addition was completed, reflux was continued for 3h and left overnight. Refined by fractional distillation.
Acetyl bromide may also be prepared by reaction between phosphorus tribromide and acetic acid:
3CH3COOH + PBr3 → 3 CH3COBr + H3PO3
General Description
A colorless fuming liquid with a pungent odor. Vapors irritate eyes and mucous membranes. Corrosive to metals and tissue. Density 13.9 lb / gal.
Reactivity Profile
Acetyl bromide decomposes violently upon contact with water, steam, methanol or ethanol to form hydrogen bromide gas and acetic acid. Reacts vigorously with bases, both organic and inorganic. Incompatible with oxidizing agents and alcohols. Produces highly toxic fumes of bromine and carbonyl bromide when heated to decomposition [Sax, 9th ed., 1996, p. 34]. Vapor forms an explosive mixture with air [Kirk-Othmer, 3rd ed., Vol. 1, 1978, p. 162]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
Exposures to acetyl bromide cause abdominal pain, sore throat, cough, burning sensation, shortness of breath, and respiratory distress. Contact with the skin causes pain, redness, blisters, dermatitis, and skin burn, severe deep burns, loss of vision, shock or collapse. The occupational worker may show delayed symptoms and lung edema. The vapor is corrosive to the eyes, the skin, and the respiratory tract. The irritation caused by acetyl bromide may lead to chemical pneumonitis and pulmonary edema, and may cause burns to the respiratory tract. The target organs include the eyes, skin, and the mucous membranes.
Chemical Reactivity
Reactivity with Water: Reacts violently, forming corrosive and toxic fumes of hydrogen bromide; Reactivity with Common Materials: Attacks and corrodes wood and most metals in the presence of moisture. Flammable hydrogen gas may collect in enclosed spaces; Stability During Transport: Stable if protected from moisture; Neutralizing Agents for Acids and Caustics: Flood with water, rinse with dilute sodium bicarbonate or soda ash solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Poison by ingestion, inhalation, skin contact, and intraperitoneal routes. See also HYDROBROMIC ACID and ACETIC ACID. Violent reaction on contact with water, steam, methanol, or ethanol produces toxic and reactive HBr. When heated to decomposition it emits highly corrosive and toxic fumes of carbonyl bromide and bromine. To fight fire, use dry chemical, CO2
Potential Exposure
Acetyl bromide is used as an acetylating agent in the organic synthesis of other chemicals, pesticides, perfume, pharmaceuticals, and it is also used as a dye intermediate.
Storage
Acetyl bromide should be stored in a tightly sealed container, in a cool, dry, well-ventilated area, away from water and incompatible substances.
Shipping
UN1716 Acetyl bromide, Hazard class: 8; Labels: 8-Corrosive material
Purification Methods
Boil acetyl bromide with PBr3/Ac2O for 1hour, then distil the latter off and redistil it. Store it dry. [Burton & Degering J Am Chem Soc 62 227 1940, Beilstein 2 IV 398.] LACHRYMATORY.
Incompatibilities
Acetyl bromide Vapor may form explosive mixture with air. Instability increases as temperature rises, Contact with moisture, water, steam, alcohols cause a violent reaction releasing corrosive carbonyl bromide, hydrogen bromide, and bromine gases. Incompatible with organic solvents, ethers, oxidizers, and strong bases. Corrodes or attacks most metals and wood in the presence of moisture. Contact with combustibles may cause ignition
Waste Disposal
Dispose of contents and container to an approved waste disposal plant. All federal, state, and local environmental regulations must be observed. Slow addition to sodium bicarbonate solution in a glass or plastic container. Mix slowly in another container containing lots of water. It is inappropriate and possibly dangerous to the environment to dispose of chemical wasteby flushing them down the toilet or discarding them to the trash
Precautions
Acetyl bromide is combustible and emits irritating or toxic fumes (or gases) in a fire. It should have no contact with naked flames or water. During use, occupational workers should use protective gloves, protective clothing, a face shield or eye protection in combination with breathing protection and should not eat, drink, or smoke. Acetyl bromide decomposes on heating and produces toxic and corrosive fumes, such as hydrogen bromide and carbonyl bromide. It reacts violently with water, methanol, or ethanol to form hydrogen bromide. Acetyl bromide attacks and damages many metals in the presence of water.
Properties of Acetyl bromide
| Melting point: | -96 °C |
| Boiling point: | 75-77 °C(lit.) |
| Density | 1.663 g/mL at 25 °C(lit.) |
| vapor density | 4.3 (vs air) |
| vapor pressure | 92.2 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Store at <= 20°C. |
| solubility | Miscible with ether, chloroform, benzene and acetone. |
| form | Fuming Liquid |
| color | Clear colorless to dark yellow |
| Odor | Acrid and sharp. |
| Water Solubility | REACTS |
| Sensitive | Moisture Sensitive |
| Merck | 14,82 |
| BRN | 1697546 |
| Dielectric constant | 16.5(Ambient) |
| CAS DataBase Reference | 506-96-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetyl bromide(506-96-7) |
| EPA Substance Registry System | Acetyl bromide (506-96-7) |
Safety information for Acetyl bromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acetyl bromide
| InChIKey | FXXACINHVKSMDR-UHFFFAOYSA-N |
Acetyl bromide manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Acetyl bromide, 98% CAS 506-96-7View Details
Acetyl bromide, 98% CAS 506-96-7View Details
506-96-7 -
 Acetyl bromide, GR 99%+ CAS 506-96-7View Details
Acetyl bromide, GR 99%+ CAS 506-96-7View Details
506-96-7 -
 Acetyl bromide 98.00% CAS 506-96-7View Details
Acetyl bromide 98.00% CAS 506-96-7View Details
506-96-7 -
 Acetyl Bromide CAS 506-96-7View Details
Acetyl Bromide CAS 506-96-7View Details
506-96-7 -
 ACETYL BROMIDE For Synthesis CAS 506-96-7View Details
ACETYL BROMIDE For Synthesis CAS 506-96-7View Details
506-96-7 -
 Acetyl Bromide CAS Number 506-96-7, Grade Standard: Industrial, Packaging Type: CarboyView Details
Acetyl Bromide CAS Number 506-96-7, Grade Standard: Industrial, Packaging Type: CarboyView Details
506-96-7 -
 Acetyl BromideView Details
Acetyl BromideView Details
506-96-7 -
 Acetyl BromideView Details
Acetyl BromideView Details
506-96-7
