LHRH
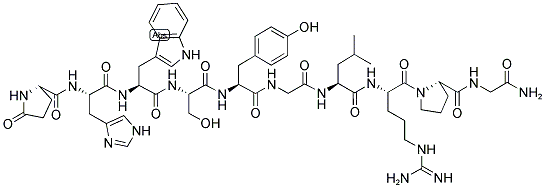
Synonym(s):[Gly-OH10]-LH-RH;GnRH;Gonadotropin-releasing hormone;LH-RH;pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly
- CAS NO.:9034-40-6
- Empirical Formula: C55H75N17O13
- Molecular Weight: 1182.29
- MDL number: MFCD00167538
- EINECS: 232-895-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
What is LHRH?
Description
Gonadotropin-releasing hormone is a decapeptide that is produced in neurosecretory cells within the hypothalamus. GnRH stimulates the synthesis and release of luteinizing hormone (LH) and folliclestimulating hormone (FSH) from the anterior pituitary and also controls reproductive behavior, thus serving as a central regulator of reproduction in vertebrates. It is used not only as a fertility drug but also as an antifertility drug.
Indications
GnRH (gonadorelin, luteinizing hormone–releasing hormone) is a decapeptide that stimulates production of LH and FSH. It is released in bursts from the hypothalamus at regular intervals, about every 2 hours, although in women the interval may lengthen in the luteal end of the menstrual cycle.The pituitary gland responds to these regular pulses by producing LH and FSH. The pattern of LH and FSH in cycling women, including the large burst of LH release before ovulation, can be stimulated by regular administration of GnRH pulses. The large burst of LH from the pituitary gland appears to be induced by feedback through estradiol and other products of the gonads that change the response of the pituitary gland to the GnRH pulses rather than by large changes in the amounts of GnRH secreted. The stimulatory response to GnRH depends on pulsatile administration and the timing of the pulses. Continual administration of GnRH does not have the same effects as pulsatile administration; although production of LH and FSH is stimulated initially, it is suppressed within a few days. Part of this desensitization to GnRH is caused by a decrease in the number of pituitary receptors for GnRH; additional postreceptor mechanisms are also important in this complete suppression.
Indications
Oxytocin (Pitocin, Syntocinon) is a cyclic 8–amino acid
peptide that is synthesized in the paraventricular nucleus
of the hypothalamus and transported within hypothalamic
neurons (in association with neurophysin)
to the posterior pituitary for storage. Its mechanism of
action involves the direct stimulation of oxytocin receptors
found on the myometrial cells. Oxytocin circulates
unbound in the plasma, where it has a half-life of approximately
15 minutes. It is primarily inactivated in the
kidneys and liver.
Oxytocin (Pitocin, Syntocinon) causes milk release (letdown)
by stimulating contraction of the myoepithelial
cells of the milk ducts in lactating mammary glands; this
forces milk from the alveoli of the breast. Oxytocin release
is stimulated by suckling and by auditory and visual
stimuli, such as a baby’s cry.Oxytocin is available as
a nasal spray, which is used as an aid to lactation when
milk ejection is impaired.
Definition
ChEBI: Gonadorelin is a ten-membered synthetic oligopeptide comprising pyroglutamyl, histidyl, tryptophyl, seryl, tyrosyl, glycyl, leucyl, arginyl, prolyl and glycinamide residues joined in sequence. It has a role as a gonadotropin releasing hormone agonist. It is an oligopeptide and a peptide hormone.
Biological Functions
Gonadotropin-releasing hormone (GnRH) is a decapeptide that causes the release of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), from the anterior pituitary gland, but not in equal amounts (FSH release is partially inhibited by the gonadal protein inhibin). Therefore, GnRH is intimately involved in the control of both male and female reproduction. Medicinal chemists have capitalized on the relatively simple decapeptide structure of GnRH by preparing many analogues as potential fertility and antifertility agents, several of which are commercially available, especially those that are referred to as superagonists. It is known that GnRH can be degraded by enzymatic cleavage between Tyr5- Gly6 and Pro9-Gly10. Structure–activity relationship studies of GnRH analogues have shown that when Gly6 is replaced with certain D-amino acids, as well as with changes in the peptide C terminus, they generally are less susceptible to proteolytic enzymes, resulting in a longer lasting action. For that reason, they are referred to as superagonists. Furthermore, when these D-amino acids at position 6 are hydrophobic, the half-life is enhanced.
Mechanism of action
In physiological doses, GnRH agonists are able to induce ovulation and spermatogenesis by increasing LH and FSH levels and the resulting sex steroid levels, as does the normal hormone. In larger pharmacological (therapeutic) doses, however, GnRH agonists, especially the superagonists, block implantation of the fertilized egg, cause luteolysis of the corpus luteum, and can act as postcoital contraceptive agents (although not approved for this latter use). This paradoxical antifertility effect seen with the superagonists has been attributed to the fact that GnRH must be administered in a low-dose, pulsatile manner for it to be therapeutically effective as a fertility agent. Natural GnRH release from the hypothalamus occurs in a pulsatile manner. When GnRH or, especially, a superagonist is administered in pharmacological doses each day, LH and FSH levels will initially rise but then begin to fall after a few days because of target tissue desensitization/downregulation of pituitary GnRH receptors. The continued use of these agents in a nonpulsatile manner will result in a drastic drop of the gonadal steroid levels to near castrate levels in both males and females, thereby giving rise to their use in such conditions as precocious puberty, endometriosis, and advanced metastatic breast and prostate carcinoma.Typically, however, the GnRH superagonists take approximately 2 weeks to finally desensitize the GnRH receptors, and during this time, there is a transient rise in LH and FSH levels, which often results in an initial “flare-up” of the original symptoms.
Clinical Use
Oxytocin is generally considered to be the drug of choice for inducing labor at term. In combination with amniotomy, oxytocin is highly successful in inducing and augmenting labor. When given oxytocin, approximately 80% of patients with documented labor disorders progress into labor and deliver vaginally. It has also been used following incomplete abortion after 20 weeks of gestation (although use of prostaglandins may be preferred in this instance), and it may be used after fullterm delivery to prevent or control uterine hemorrhage. Oxytocin in high doses is used to induce abortion. An oxytocin challenge test (an assessment of the fetal heart rate in response to oxytocin-induced contractions) can be performed in certain high-risk (e.g., those with hypertension, diabetes, preeclampsia) obstetrical patients as a measure of fetal well-being.
Clinical Use
Because mGnRH has a short half-life of several minutes, a number of GnRH analogs with high potency and long half-life have been synthesized. The effects of GnRH and its analogs are dependent on the dose and method of administration. Low doses of GnRH delivered in a pulsatile fashion restore fertility in hypogonadal patients. High doses of GnRH or continuous administration first results in an increase in LH and FSH secretion, followed by a decrease in LH and FSH levels due to desensitization, then by a decline in gonadal steroid levels. The administration of antagonists interrupts GnRH-dependent LH and FSH secretion through competition with endogenous GnRH, but the doses required are much higher that the desensitizing agonists. mGnRH is available as gonadorelin for veterinary use. Various GnRH agonists are used in the treatment of hormoneresponsive cancers such as prostate and breast cancers, estrogen-dependent conditions such as endometriosis, and precocious puberty. They are also widely used in ART (assisted reproductive technology including IVFET, in vitro fertilization, and embryo transfer) to block the endogenous LH surge in the controlled ovarian stimulation
Side Effects
Inappropriate use of oxytocin can lead to uterine rupture, anaphylactoid and other allergic reactions, and possibly maternal death. Prolonged stimulation of uterine contractions can result in the following fetal adverse reactions: persistent uteroplacental insufficiency, sinus bradycardia, premature ventricular contractions, other arrhythmias, and fetal death. Prolonged use of oxytocin can lead to water intoxication secondary to the antidiuretic hormone–like effects of oxytocin. Maternal and fetal cardiovascular parameters should be monitored during oxytocin administration.
Safety Profile
An experimental teratogen. Human reproductive effects in women by subcutaneous route: menstrual cycle changes and other unspecified effects. Experimental reproductive effects. Used in the treatment of oligospermia and male inferthty. See also LUTEINIZING HORMONE and other luteinizing hormone-releasing hormone entries.
Properties of LHRH
| Boiling point: | 834.95°C (rough estimate) |
| alpha | D25 -50° (1% acetic acid) |
| Density | 1.1147 (rough estimate) |
| refractive index | 1.6200 (estimate) |
| storage temp. | -15°C
|
| EPA Substance Registry System | Luteinizing hormone-releasing factor (9034-40-6) |
Safety information for LHRH
Computed Descriptors for LHRH
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


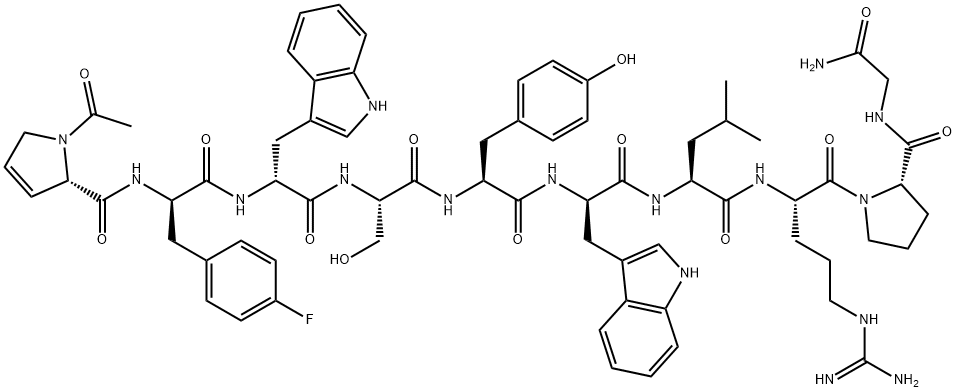
![[D-TRP6]-LHRH FRAGMENT (1-6) AMIDE](https://img.chemicalbook.in/)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1