A77 1726
- CAS NO.:163451-81-8
- Empirical Formula: C12H9F3N2O2
- Molecular Weight: 270.21
- MDL number: MFCD00910058
- EINECS: 642-273-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
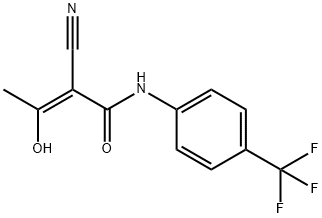
What is A77 1726?
Absorption
After oral administration of teriflunomide, maximum plasma concentrations are reached, on average, in 1-4 hours.
Toxicity
Teriflunomide is contraindicated in pregnant women or women of childbearing age due to the risk of teratogenicity. Teriflunomide is also contraindicated in severe hepatic impairment due to reports of hepatotoxicity, hepatic failure, and death.
Description
In September 2012, the US FDA approved teriflunomide (also referred to as HMR-1726) as a therapy for patients with relapsing forms of multiple sclerosis (MS). Teriflunomide is the second approved oral treatment option for MS, after Gilenya? which was approved in 2010. Teriflunomide, which is the active metabolite of leflunomide (a marketed drug for the treatment of rheumatoid and psoriatic arthritis), is a non?competitive and selective inhibitor of dihydroorotate dehydrogenase (DHODH), the rate-limiting enzyme in the de novo synthesis of pyrimidines. Although the net effect of inhibition of DHODH by teriflunomide and its therapeutic effect in MS are not clear, it is hypothesized that by inhibiting de novo pyrimidine synthesis, the effector functions of activated lymphocytes are suppressed, thus dampening the effect of an overactive immune system.
Description
A-771726 is an active metabolite of the prodrug leflunomide that inhibits dihydroorotate dehydrogenase (DHODH; IC50 = 0.23 μM). A-771726 inhibits the production of prostaglandin E2 (PGE2; ) in TNF-α- or IL-1α-stimulated isolated human synoviocytes (IC50s = 7 and 3 μM, respectively). It inhibits the proliferation of isolated human peripheral blood mononuclear cells (PBMCs) when used at concentrations of 25 and 100 μM. A-771726 (3 and 10 mg/kg) delays disease onset and decreases neurological deficits in a rat model of experimental autoimmune encephalomyelitis (EAE) induced by complete Freund’s adjuvant (CFA) and M. tuberculosis. Formulations containing teriflunomide have been used in the treatment of multiple sclerosis.
Chemical properties
White Solid
Originator
Genzyme (United States)
The Uses of A77 1726
The active metabolite of Leflunomide, a potent disease-modifying antirheumatic drug used in the treatment of rheumatoid arthritis. LEF-M interferes with dendritic cell function.
The Uses of A77 1726
A-771726 is the active metabolite of leflunomide, a prodrug approved by the FDA for treatment of rheumatoid arthritis. A-771726 reversibly inhibits dihydroorotate dehydrogenase, the rate-limiting step in the de novo synthesis of pyrimidines. It prevents activated lymphocytes from accumulating sufficient pyrimidines to support DNA synthesis (IC50s = 0.09, 3.5, and 12.5 μM for rat, mouse, and human, respectively). At higher doses, A-771726 inhibits tyrosine kinases responsible for early T cell and B cell signaling in the G0/G1 phase of the cell cycle. A-771726 has also been shown to inhibit the production of prostaglandin E2 in synoviocytes activated by TNF-α and IL-1α (IC50s = 7 and 3 μM, respectively) as well as inhibit MMP-1 and IL-6 production at concentrations >10 μM.[Cayman Chemical]
The Uses of A77 1726
Teriflunomide has been used as a dihydroorotate dehydrogenase (DHODH) inhibitor.
What are the applications of Application
A77 1726 is an active metabolite of leflunomide that inhibits Cox-2
Background
Teriflunomide is the active metabolite of leflunomide, and it acts as an immunomodulatory agent by inhibiting pyrimidine synthesis. It is marketed under the name Aubagio? and is indicated for the treatment of multiple sclerosis, specifically relapsing forms. The FDA label states an important warning about the risk of hepatoxicity and teratogenicity for patients using teriflunomide.
Indications
Used in the treatment of relapsing forms of multiple sclerosis (MS).
Definition
ChEBI: An enamide obtained by formal condensation of the carboxy group of (2Z)-2-cyano-3-hydroxybut-2-enoic acid with the anilino group of 4-(trifluoromethyl)aniline. Used for the treatment of relapsing forms of multiple sclerosis and rheumatoid arthritis.
brand name
Aubagio
General Description
Teriflunomide, marketed under the trade name Aubagio, is the active metabolite of leflunomide, an immunosuppressive disease-modifying antirheumatic drug, used in active moderate-to-severe rheumatoid arthritis and psoriatic arthritis. An analytical reference standard for use in LC/MS or GC/MS applications including clinical and diagnostic testing such as therapeutic drug monitoring assays.
Biochem/physiol Actions
Teriflunomide is an orally available anti-inflammatory immunomodulator. It blocks the activity of dihydroorotate dehydrogenase, preventing pyrimidine synthesis and T and B cell proliferation and function. Teriflunomide has been used to treat rheumatoid arthritis and was recently approved for multiple sclerosis.
Pharmacokinetics
Teriflunomide is an immunomodulatory agent that decreases the amount of activated CNS lymphocytes, which results in anti-inflammatory and antiproliferative effects.
Metabolism
Teriflunomide mainly undergoes hydrolyis to minor metabolites. Other minor metabolic pathways include oxidation, N-acetylation and sulfate conjugation. Teriflunomide is not metabolized by CYP450 or flavin monoamine oxidase.
References
[1] yao h w, li j, chen j q, et al. a 771726, the active metabolite of leflunomide, inhibits tnf-α and il-1 from kupffer cells[j]. inflammation, 2004, 28(2): 97-103.
[2] breedveld f c, dayer j m. leflunomide: mode of action in the treatment of rheumatoid arthritis[j]. annals of the rheumatic diseases, 2000, 59(11): 841-849.
[3] burger d, begué‐pastor n, benavent s, et al. the active metabolite of leflunomide, a77 1726, inhibits the production of prostaglandin e2, matrix metalloproteinase 1 and interleukin 6 in human fibroblast‐like synoviocytes[j]. rheumatology, 2003, 42(1): 89-96.
[4] davis j p, cain g a, pitts w j, et al. the immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase[j]. biochemistry, 1996, 35(4): 1270-1273.
Properties of A77 1726
| Melting point: | 229-232°C |
| Boiling point: | 410.8±45.0 °C(Predicted) |
| Density | 1.424±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble5mg/mL, clear (warmed) |
| form | powder |
| pka | 5.20±0.50(Predicted) |
| color | white to beige |
| λmax | 203nm(MeOH)(lit.) |
| Merck | 14,9165 |
| Stability: | Hygroscopic |
Safety information for A77 1726
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for A77 1726
| InChIKey | UTNUDOFZCWSZMS-YFHOEESVSA-N |
A77 1726 manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](https://img.chemicalbook.in/CAS/GIF/24522-30-3.gif)
You may like
-
 A77 1726 95% CAS 163451-81-8View Details
A77 1726 95% CAS 163451-81-8View Details
163451-81-8 -
 Teriflunomide CAS 163451-81-8View Details
Teriflunomide CAS 163451-81-8View Details
163451-81-8 -
 Teriflunomide CAS 163451-81-8View Details
Teriflunomide CAS 163451-81-8View Details
163451-81-8 -
 Teriflunomide CAS 163451-81-8View Details
Teriflunomide CAS 163451-81-8View Details
163451-81-8 -
 Teriflunomide solution CAS 163451-81-8View Details
Teriflunomide solution CAS 163451-81-8View Details
163451-81-8 -
 163451-81-8 Leflunomide EP Impurity B Leflunomide USP RC B ; (Z)-2-cyano-3-hydroxy-N-(4- (trifluoromethyl) phenyl)but-2-enamide 96%View Details
163451-81-8 Leflunomide EP Impurity B Leflunomide USP RC B ; (Z)-2-cyano-3-hydroxy-N-(4- (trifluoromethyl) phenyl)but-2-enamide 96%View Details
163451-81-8 -
 163451-81-8 99%View Details
163451-81-8 99%View Details
163451-81-8 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1