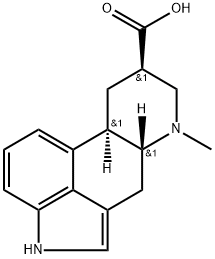
9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID
- CAS NO.:82-58-6
- Empirical Formula: C16H16N2O2
- Molecular Weight: 268.31
- MDL number: MFCD00133297
- EINECS: 201-431-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:41:45
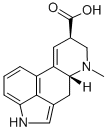
What is 9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID?
Description
A precursor of the semisynthetic ergot derivatives, but having no biological activity itself. It is subject to controls under the Controlled Substances Act of 1970 in the United States, since it is the immediate precursor for the synthesis of LSD. Lysergic acid is obtained by hydrolysis of ergot alkaloids, either obtained from grains infected with Claviceps or, more commonly, by fermentation in submerged culture.
Description
Robert Burns Woodward is generally considered to be the greatest synthetic organic chemist of the 20th century. From the 1940s to the 1970s, he led the syntheses of numerous natural products and pharmaceuticals. In 1965, Woodward received the Nobel Prize in Chemistry for this body of work.
Over the years, Molecule of the Week has featured several “Woodward molecules”, including?quinine,?cholesterol,?cortisone,?strychnine, and?chlorophyll. Here are four more of those important compounds with the dates their syntheses were reported.
Reserpine?(1958) was originally isolated from?Rauwolfia serpentina?in 1952. It was once used as a treatment for high blood pressure and psychotic episodes, but it has been replaced by newer drugs with fewer side effects.
Lysergic acid?(1956) is the biosynthetic precursor for several ergoline alkaloids. Its most notorious end product is LSD (lysergic acid diethylamide), a psychedelic drug promoted by Timothy Leary in the 1960s.
Cephalosporin C?(1966) is a member of the cephalosporin family of β-lactam antibiotics. They were discovered in 1945 in?Acremonium?fungus species.
Colchicine?(1963), primarily used to treat gout, dates to prehistoric times when its source, the?Colchicum autumnale?(crocus) plant, was used to treat rheumatism and swelling.
Woodward’s crowning synthetic accomplishment was vitamin B12?(cobalamin) in 1972. For this work, he collaborated with Swiss chemist Albert Eschenmoser and more than 100 graduate students and postdoctoral fellows. Each of the research groups contributed a portion of the molecule.
Woodward’s achievements in physical organic chemistry were also ground-breaking. Using molecular orbital theory, he and theoretical chemist Roald Hoffmann formulated rules that govern pericyclic reactions, such as cycloadditions. Hoffmann was awarded the 1981 Nobel Prize in chemistry for the Woodward–Hoffman rules, 2 years after Woodward’s death.
Bethany Halford’s article in this week’s issue of C&EN?commemorates Woodward’s career.
Chemical properties
beige to brown powder
The Uses of 9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID
Labelled Lysergic acid. Lysergic acid and iso-lysergic acid are the main cleavage products formed on alkaline hydrolysis of the alkaloids which are characteristic of ergot. Potent hallucinogen; non-selective serotonin receptor agonist. Controlled substanc
The Uses of 9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID
Lysergic acid and iso-lysergic acid are the main cleavage products formed on alkaline hydrolysis of the alkaloids which are characteristic of ergot. Potent hallucinogen; non-selective serotonin receptor agonist. Controlled substance.
Definition
ChEBI: An ergoline alkaloid comprising 6-methylergoline having additional unsaturation at the 9,10-position and a carboxy group at the 8-position.
Synthesis Reference(s)
Journal of the American Chemical Society, 78, p. 3087, 1956 DOI: 10.1021/ja01594a039
Flammability and Explosibility
Not classified
Purification Methods
It crystallises from water as a hydrate. The methyl ester crystallises from C6H6 and has m 168o; the amide [478-94-4] has m 242o(dec) (from MeOH) and []546 +15o (c 0.5, pyridine).The (-)-hydrochloride has m 208-210o(dec, from MeOH). [Kornfeld et al. J Am Chem Soc 7 6 5256 1954,Kornfeld et al. J Am Chem Soc 78 3087 1956, Beilstein 25 III/IV 934,]
Properties of 9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID
| Melting point: | >170°C (dec.) |
| Boiling point: | 411.48°C (rough estimate) |
| alpha | D20 +40° (c = 0.5 in pyridine) |
| Density | 1.1028 (rough estimate) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.6240 (estimate) |
| storage temp. | Controlled Substance, -20°C Freezer |
| pka | 3.44, 7.68(at 25℃) |
| Water Solubility | 267mg/L at 20℃ |
Safety information for 9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID
Computed Descriptors for 9,10-DIDEHYDRO-6-METHYL-ERGOLINE-8-CARBOXYLIC ACID
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1