9,10-Dibromoanthracene
- CAS NO.:523-27-3
- Empirical Formula: C14H8Br2
- Molecular Weight: 336.02
- MDL number: MFCD00001244
- EINECS: 208-342-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
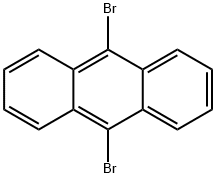
What is 9,10-Dibromoanthracene?
Description
9,10-Dibromoanthracene is an aryl dihalide that until recently had no noteworthy properties or uses. It was first synthesized in 1923 by Ian (formerly Isidor) M. Heilbron and John S. Heaton, chemists working at the University of Liverpool. The molecule forms an addition compound with the explosive 1,3,5-trinitrobenzene.
But early this year, Leo Gross and colleagues at IBM Research Zurich and the University of Santiago de Compostela (Spain) used 9,10-dibromoanthracene to demonstrate that?a chemical reaction can be observed at the molecular level. They combined scanning tunneling microscopy (STM) and atomic force microscopy to watch voltage pulses from an STM probe break one C–Br bond to form a free radical and then the second bond to form a diradical. Another pulse converted the diradical to a bicyclic diene–diyne.
The researchers also showed that their procedure can be used to reverse the last reaction to re-form the diradical. Chemists who study reaction intermediates hail this study as a breakthrough for directly observing chemical bonding changes in a reaction.
Description
9,10-Dibromoanthracene is an organic chemical compound containing anthracene with two bromine atoms substituted on its central ring. As a symmetrically brominated anthracene derivative, it is bearing functional groups to extend its conjugation further mainly via C-C bond forming i.e. Stille and Suzuki coupling reactions. It is notable in that it was the first single molecule to have a chemical reaction observed by an atomic force microscope and scanning tunneling microscopy. It is a low-molecular-weight organic semiconductor. It can used as an initiator in visible-spectrum solar-light-mediated benzylic C–H oxygenation through solar light or the blue LED activate[1-2].
Chemical properties
Yellow to yellow-green fluffy powder. Melting point 226°C. It can be sublimated and oxidized to generate anthraquinone. It is soluble in hot benzene and hot toluene, but only slightly soluble in alcohol, ether, and cold benzene. It is insoluble in water.
The Uses of 9,10-Dibromoanthracene
9,10-Dibromoanthracene is a dibrominated polycyclic aromatic hydrocarbon (PAH). 9,10-Dibromoanthracene is often used as an energy acceptor and activator in reactions that produce chemiluminescence.
Preparation
9,10-Dibromoanthracene is synthesized by the bromination of anthracene. The bromination reaction is carried out at room temperature using carbon tetrachloride as a solvent. Using 80-85% anthracene as raw material, adding bromine to react for half an hour, the yield is 83-88%.
Origin
9,10-Dibromoanthracene was first synthesized in 1923 by Ian (formerly Isidor) M. Heilbron and John S. Heaton, chemists working at the University of Liverpool.
Purification Methods
Recrystallise it from toluene, xylene or CCl4 (yellow needles), and sublime it in a vacuum [Johnston et al. J Am Chem Soc 109 1291 1987]. [Heilbron & Heaton Org Synth Coll Vol I 207 1941, Beilstein 5 H 665.]
References
[1] Santra S, et al. Visible-Spectrum Solar-Light-Mediated Benzylic C–H Oxygenation Using 9,10-Dibromoanthracene As an Initiator. The Journal of Organic Chemistry, 2020; 86: 1164–1171.
[2] Watanabe M. Formation of aligned needle-like crystals of 9,10-dibromoanthracene in small droplets. Journal of Applied Polymer Science, 2023; 140: e54515.
Properties of 9,10-Dibromoanthracene
| Melting point: | 223-224 °C (lit.) |
| Boiling point: | 342.21°C (rough estimate) |
| Density | 1.7167 (estimate) |
| refractive index | 1.6300 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform |
| form | Fluffy Powder |
| color | Yellow to yellow-green |
| Water Solubility | Soluble in water. |
| Merck | 14,3018 |
| BRN | 1912109 |
| CAS DataBase Reference | 523-27-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Anthracene, 9,10-dibromo-(523-27-3) |
| EPA Substance Registry System | Anthracene, 9,10-dibromo- (523-27-3) |
Safety information for 9,10-Dibromoanthracene
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for 9,10-Dibromoanthracene
| InChIKey | BRUOAURMAFDGLP-UHFFFAOYSA-N |
9,10-Dibromoanthracene manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 523-27-3 9,10-DIBROMOANTHRACENE 98%View Details
523-27-3 9,10-DIBROMOANTHRACENE 98%View Details
523-27-3 -
 523-27-3 99%View Details
523-27-3 99%View Details
523-27-3 -
 523-27-3 9,10-Dibromoanthracene, 98% 99%View Details
523-27-3 9,10-Dibromoanthracene, 98% 99%View Details
523-27-3 -
 9,10-Dibromoanthracene, 98% CAS 523-27-3View Details
9,10-Dibromoanthracene, 98% CAS 523-27-3View Details
523-27-3 -
 9,10-Dibromoanthracene CAS 523-27-3View Details
9,10-Dibromoanthracene CAS 523-27-3View Details
523-27-3 -
 9,10-Dibromoanthracene CAS 523-27-3View Details
9,10-Dibromoanthracene CAS 523-27-3View Details
523-27-3 -
 9,10-Dibromoanthracene CAS 523-27-3View Details
9,10-Dibromoanthracene CAS 523-27-3View Details
523-27-3 -
 9,10-DibromoanthraceneView Details
9,10-DibromoanthraceneView Details
523-27-3
