9-Bromoanthracene
- CAS NO.:1564-64-3
- Empirical Formula: C14H9Br
- Molecular Weight: 257.13
- MDL number: MFCD00001243
- EINECS: 216-359-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:13:45
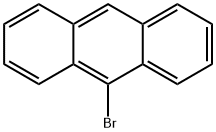
What is 9-Bromoanthracene?
Description
9-bromoanthracene is a kind of bromine-derived anthracene. It is known to be able to reversibly photodimerize in a head-to tail fashion upon irradiation by long-wavelength ultraviolet light. The photodimers of 9-bromoanthracene are suitable to be used as alkyl halide initiators in the atom transfer radical polymerization (ATRP) reactions. It is also used as the intermediate for the preparation of the 9-substitued form of the polycyclic aromatic hydrocarbon (PAH) anthracene.
Chemical properties
white to light yellow crystal powder. boiling point 190°C (0.16kPa, sublimation), relative density 1.409. soluble in acetic acid; carbon disulfide.
The Uses of 9-Bromoanthracene
9-Bromoanthracene acts as an intermediate in the preparation of 9-substituted derivative of the polycyclic aromatic hydrocarbon (PAH) anthracene.
Preparation
Anthracene (5 g, 28.05 mmol) was dissolved in CHCl3. Then N-bromosuccinimide (NBS,4.99 g, 28.05 mmol) was added in batches away from light, and the reaction solution was continuously stirred for 12 h. The resulting mixture was stirred for another 30 min with appropriate water, and extracted with CH2Cl2. The CH2Cl2 solution was dried over anhydrous MgSO4. After removing CH2Cl2 solvent, the residue was recrystallized from anhydrous ethanol to give 4.78 g (66.3 %) of a green-yellow needle solid. 1H NMR (500 MHz, CDCl3) δ 8.55 (d, J = 8.9 Hz, 2H), 8.48 (s, 1H), 8.03 (d, J = 8.4 Hz, 2H), 7.67 – 7.60 (m, 2H), 7.56 – 7.51 (m, 2H). EI-MS (m/z): Calculated for C14H9Br: 257.13. Found [M+ ]: 255.96.
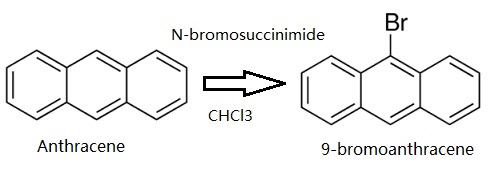
Synthesis of 9-bromoanthracene
Synthesis Reference(s)
The Journal of Organic Chemistry, 57, p. 2740, 1992 DOI: 10.1021/jo00035a038
Purification Methods
Crystallise 9-bromoanthracene from MeOH or EtOH followed by sublimation in vacuo. [Masnori et al. J Am Chem Soc 108 126 1986, Beilstein 5 IV 2295.]
References
Cohen, Nicole A., et al. Macromolecular Chemistry and Physics 210.3 ‐4 (2009): 263-268.
Xu, Xiaoming, Wenzhe Lu, and Richard B. Cole. Analytical chemistry 68.23 (1996): 4244-4253.
Dang, Hung, and Miguel A. Garcia-Garibay. Journal of the American Chemical Society 123.2 (2001): 355-356.
Properties of 9-Bromoanthracene
| Melting point: | 97-100 °C (lit.) |
| Boiling point: | 303.85°C (rough estimate) |
| Density | 1.4251 (rough estimate) |
| refractive index | 1.6404 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Powder |
| color | Yellow |
| Water Solubility | Insoluble in water. |
| BRN | 1869747 |
| InChI | InChI=1S/C14H9Br/c15-14-12-7-3-1-5-10(12)9-11-6-2-4-8-13(11)14/h1-9H |
| CAS DataBase Reference | 1564-64-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 9-Bromoanthracene(1564-64-3) |
Safety information for 9-Bromoanthracene
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for 9-Bromoanthracene
| InChIKey | ZIRVQSRSPDUEOJ-UHFFFAOYSA-N |
| SMILES | C1=C2C(C=C3C(=C2Br)C=CC=C3)=CC=C1 |
9-Bromoanthracene manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran






You may like
-
 1564-64-3 9-BROMOANTHRACENE 98%View Details
1564-64-3 9-BROMOANTHRACENE 98%View Details
1564-64-3 -
 9-Bromoanthracene 99%View Details
9-Bromoanthracene 99%View Details
1564-64-3 -
 9-Bromoanthracene, 95% 1564-64-3 99%View Details
9-Bromoanthracene, 95% 1564-64-3 99%View Details
1564-64-3 -
 9-Bromoanthracene CAS 1564-64-3View Details
9-Bromoanthracene CAS 1564-64-3View Details
1564-64-3 -
 9-Bromoanthracene, 98% CAS 1564-64-3View Details
9-Bromoanthracene, 98% CAS 1564-64-3View Details
1564-64-3 -
 9-Bromoanthracene CAS 1564-64-3View Details
9-Bromoanthracene CAS 1564-64-3View Details
1564-64-3 -
 9-Bromoanthracene CAS 1564-64-3View Details
9-Bromoanthracene CAS 1564-64-3View Details
1564-64-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
