9-Phenylacridine
- CAS NO.:602-56-2
- Empirical Formula: C19H13N
- Molecular Weight: 255.31
- MDL number: MFCD00092562
- EINECS: 210-020-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
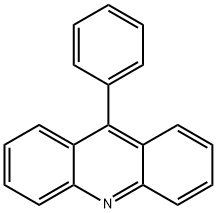
What is 9-Phenylacridine?
The Uses of 9-Phenylacridine
Research has found that 9-phenylacridine (ACPH) exhibited anticancer activity in cell lines and in vivo animal models. Bioinformatics studies have indicated that it could have topoisomerase I inhibitory activity. It could also act as a PARP 1 inhibitor and was very effective in combination with cisplatin in cell line-based assays. ACPH also satisfied all criteria for a candidate drug from the Lipinski rule and showed good cell uptake. ACPH alone was effective in the A375 melanoma cell line. ACPH could be used as a promising new photosensitizer[1-2]. 9-Phenylacridine is the parent base of chrysaniline or 3,6-diamino-9- phenylacridine, the chief constituent of the dyestuff phosphine. It is commonly used in the electronics industry as a free radical photoinitiator, significantly improving photo speed.
Description
9-Phenylacridine has antitumour activity. It can be used as a photosensitiser and can sensitise UVA-induced DNA damage in vitro and in cells. It also enhances cell killing by UVA. In addition. It is also used in photochemical research and as a raw material for organic synthesis.
Biological Activity
Hansda et al. suggested that a nontoxic dose of 9-Phenylacridine (ACPH) with UVA light can inhibit the proliferation of A375 cells by inducing DNA damage both in in vitro DNA and in cells through photosensitization. Such treatment enhanced the generation of ROS in cells, leading to lipid peroxidation and depletion of GSH. This led to cell cycle arrest at the G2/M phase to promote apoptosis through the mitochondria-mediated pathway. Although there was a slight increase in the small population of cells undergoing death through autophagy by UVA on ACPH pretreatment, primarily UVA+ACPH resulted in cell death through apoptosis. Thus, using ACPH as a photosensitizer in PDT may be contemplated. Applying ACPH topically to target cells and then illuminating them with UVA can minimize the concern of inducing damage to the surrounding normal cells [1].
References
[1] Surajit Hansda, Rita Ghosh, Gargi Ghosh. "9-phenyl acridine photosensitizes A375 cells to UVA radiation." Heliyon (2020): e04733.
[2] Rita Ghosh, Dipanjan Guha, Sudipta Bhowmik. "9-phenyl acridine exhibits antitumour activity by inducing apoptosis in A375 cells." Molecular and Cellular Biochemistry 361 1–2 (2012): 55–66.
Properties of 9-Phenylacridine
| Melting point: | 184°C |
| Boiling point: | 388.54°C (rough estimate) |
| Density | 0.9418 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| pka | 5.45±0.10(Predicted) |
| form | powder to crystal |
| color | Light yellow to Amber to Dark green |
| InChI | InChI=1S/C19H13N/c1-2-8-14(9-3-1)19-15-10-4-6-12-17(15)20-18-13-7-5-11-16(18)19/h1-13H |
| CAS DataBase Reference | 602-56-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Acridine, 9-phenyl-(602-56-2) |
| EPA Substance Registry System | Acridine, 9-phenyl- (602-56-2) |
Safety information for 9-Phenylacridine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 9-Phenylacridine
| InChIKey | MTRFEWTWIPAXLG-UHFFFAOYSA-N |
| SMILES | C1C2C(=NC3C(C=2C2=CC=CC=C2)=CC=CC=3)C=CC=1 |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 9-Phenylacridine CAS 602-56-2View Details
9-Phenylacridine CAS 602-56-2View Details
602-56-2 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
