8-PRENYLNARINGENIN
- CAS NO.:53846-50-7
- Empirical Formula: C20H20O5
- Molecular Weight: 340.37
- MDL number: MFCD00272175
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:05
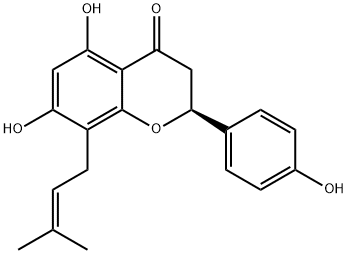
What is 8-PRENYLNARINGENIN?
The Uses of 8-PRENYLNARINGENIN
(-)-8-Prenylnaringin is a prenylflavonoid, a class on non-steroidal phytoestrogen that mimicks and/or modulating endogenous estrogens via estrogen receptor binding.
Definition
ChEBI: Sophoraflavanone B is a trihydroxyflavanone that is (S)-naringenin having a prenyl group at position 8. It has a role as a platelet aggregation inhibitor and a plant metabolite. It is a trihydroxyflavanone, a member of 4'-hydroxyflavanones and a (2S)-flavan-4-one. It is functionally related to a (S)-naringenin. It is a conjugate acid of a sophoraflavanone B(1-).
Biological Activity
8-prenylnaringenin is an estrogen receptor inhibitor.the two estrogen receptors erα and erβ belong to the nuclear receptor superfamily and are ligand-regulated transcription factors accounting for mediating the physiological effects of the steroid hormone 17α-estradiol. er is an established target for the development of synthetic ligands for therapeutic applications.
in vitro
previous study identified 8-prenylnaringenin as a potent phytoestrogen in hops, which had an activity greater than other known plant estrogens. the estrogenic activity of 8-prenylnaringenin was reflected in its relative binding affinity to estrogen receptors from rat uteri. in addition, the presence of 8-prenylnaringenin in hops might explane the accounts of menstrual disturbances in female hop workers [1].
in vivo
previous animal data demonstrated that 8-prenylnaringenin treatment could result in ampk signaling pathway activation, therefore suppressing lipogenesis. the consumption of 8-prenylnaringenin was able to prevent body weight gain and improve plasma lipid profile, with significant improvement of insulin resistance and glucose tolerance. moreove, it was found that 8-prenylnaringenin-enriched diet could ameliorate diabetic-associated metabolic disturbances via regulating glucose and lipid pathways [2].
References
[1] milligan sr, kalita jc, heyerick a, rong h, de cooman l, de keukeleire d. identification of a potent phytoestrogen in hops (humulus lupulus l.) and beer. j clin endocrinol metab. 1999 jun;84(6):2249-52.
[2] costa r et al. xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. j nutr biochem. 2017 apr 6;45:39-47.
Properties of 8-PRENYLNARINGENIN
| Boiling point: | 597.6±50.0 °C(Predicted) |
| Density | 1.314±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO |
| form | neat |
| pka | 7.70±0.40(Predicted) |
| form | Solid |
| color | White to off-white |
| BRN | 5611472 |
| InChI | InChI=1S/C20H20O5/c1-11(2)3-8-14-15(22)9-16(23)19-17(24)10-18(25-20(14)19)12-4-6-13(21)7-5-12/h3-7,9,18,21-23H,8,10H2,1-2H3/t18-/m0/s1 |
Safety information for 8-PRENYLNARINGENIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 8-PRENYLNARINGENIN
| InChIKey | LPEPZZAVFJPLNZ-SFHVURJKSA-N |
| SMILES | [C@H]1(C2=CC=C(O)C=C2)OC2=C(C/C=C(/C)\C)C(O)=CC(O)=C2C(=O)C1 |
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4