7,8-Dihydroxyflavone
- CAS NO.:38183-03-8
- Empirical Formula: C15H10O4
- Molecular Weight: 254.24
- MDL number: MFCD00006836
- EINECS: 253-812-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
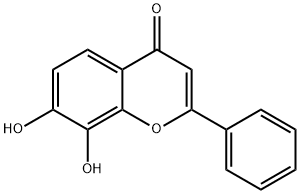
What is 7,8-Dihydroxyflavone?
Description
7,8-Dihydroxyflavone(7,8-DHF) is a naturally occurring flavone found in the tree Godmania aesculifolia. It is a selective tyrosine kinase receptor B(TrkB) agonist that has neurotrophic effects in various neurological diseases such as stroke and Parkinson's disease. 7,8-Dihydroxyflavone binds to the TrkB receptors in a similar way to BDNF, or brain derived neurotrophic factor. When the TrkB receptor is activated, neuroprotective and neurogenetive effects occur in neurons, which tend to affect the dendrites. Dendrites are what reach out from the neurons into the synapse to participate in signaling with other neurons. 7,8-DHF has been shown to promote the growth of these dendrites, and to help restore communication between neurons in animal models.
Efficacy
7,8-Dihydroxyflavone (7,8-DHF) is a naturally occurring flavonoid produced by several plants, including the weed Tridax procumbens (coalbuttons or tridax daisy) and the tree Godmania aesculifolia, which are found in the Western Hemisphere tropics, and trees in the widespread Primula genus. It has shown efficacy against several nervous-system diseases, including Alzheimer’s, Parkinson’s, and Huntington’s.
As research continues on 7,8-DHF as a treatment for these diseases, a recent discovery shows that it might be useful for combating another nervous-system scourge. Work by Tomás R Guilarte at Florida International University (Miami) and colleagues there and at New York Medical College (Valhalla) and Columbia University (New York City) shows that the compound may repair damage to the brain caused by lead poisoning.
Chemical properties
White crystalline powder or Yellow Solid, soluble in methanol, ethanol, DMSO and other organic solvents, from the dried root of Pulsatilla chinensis (Bge.) Regel.
The Uses of 7,8-Dihydroxyflavone
7,8-Dihydroxyflavone is disubstituted flavone that acts as a small-molecule TrkB agonist. 7,8-Dihydroxyflavone has been shown to reverse memory deficits and BACE1 elevation in a mouse model of Alzheimer''s disease. 7,8-Dihydroxyflavone may also provide a therapeutic option in the treatment of other neorological disorders such as Rett syndrome and PTSD. It has recently been shown to counteract the inhibition of NMDA receptor caused by lead poisoning and confer protective effects in live rats.
Definition
ChEBI: 7,8-dihydroxyflavone is a dihydroxyflavone that is flavone substituted by hydroxy groups at positions 7 and 8. A dihydroxyflavone that is flavone substituted by hydroxy groups at positions 7 and 8. A naturally occurring flavonoid produced by several plants, including the weed Tridax procumbens (coalbuttons or tridax daisy) and the tree Godmania aesculifolia, In animal models, it has shown efficacy against several diseases of the nervous system, including Alzheimer's, Parkinson's, and Huntington's. It has a role as a plant metabolite, a tropomyosin-related kinase B receptor agonist, an antidepressant, an antioxidant and an antineoplastic agent.
Biological Activity
7,8-Dihydroxyflavone (7,8-DHF) is a monophenolic flavone with diverse effects. It acts as an agonist of the neurotrophic tyrosine kinase receptor TrkB (Kd = 320 nM), protecting neurons that express TrkB from apoptosis. 7,8-DHF is neuroprotective in an animal model of Parkinson’s disease. It supports emotional learning in mice and reverses memory deficits in a mouse model of Alzheimer’s disease. It also improves motor function and extends survival in an animal model of Huntington’s disease. 7,8-DHF inhibits the cytochrome P450 aromatase (IC50 = 10 μM) and, in this way, alters estrogen metabolism. It also has antioxidant action that increases intracellular glutathione synthesis and scavenges reactive oxygen species.
Biochem/physiol Actions
7,8-Dihydroxyflavone is a selective tyrosine kinase receptor B (TrkB) receptor agonist. It manifests all the therapeutic effects of brain-derived neurotrophic factor (BDNF)—such as protecting neurons from apoptosis, inhibiting kainic acid-induced toxicity, decreasing infarct volumes in stroke, and neuroprotecting in an animal model of Parkinson′s disease—without the poor pharmacokinetic profile of BDNF limiting its therapeutic potential.
Side Effects
As with any other drug, 7,8-DHF is not without its side effects. However, there are not as many side effects associated with the use of 7,8-DHF as it is a natural product.
The following are some reported side effects associated with the use of 7,8-DHF or products that contain it:
Overstimulation
Restlessness
Dizziness
Nausea
Irritability
Trouble sleeping
Storage
Store at +4°C
Mode of action
7,8-Dihydroxyflavone (7,8-DHF, tropoflavin) is the most common and effective flavone identified in Sophora plants.
7,8-DHF mimics the effects of brain-derived neurotrophic factor (BDNF) in brain cells by activating tropomyosin-related kinase B (TrkB) receptors, the typical target of BDNF.
The therapeutic potential of BDNF is restricted due to its short half-life (less than 10 minutes) and its inability to cross the blood-brain barrier because of its large size. Unlike BDNF 7,8-DHF is able to penetrate the blood-brain barrier and enter the central nervous system (CNS) .
7,8-DHF also increases the production of Nrf2. Nrf2 increases antioxidants enzymes such as heme oxygenase 1 (HO-1) and also enzymes that repair DNA (8-oxoguanine DNA glycosylase-1 – OGG1) .
References
1) Jang et al. (2010), A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone; Proc. Natl. Acad. Sci. USA, 107 2687 2) Liu et al. (2014) Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor; J. Biol. Chem., 289 27571 3) Jiang et al. (2013) Small-molecule TrkB receptor agonist improve motor function and extend survival in a mouse model of Huntington’s disease; Hum. Mol. Genet., 22 2462 4) Zhao et al. (2016) Post-injury Treatment of 7,8-Dihydroxyflavone Promotes Neurogenesis in the Hippocampus of the Adult Mouse; J. Neurotrauma, 33 2055 5) Korkmaz et al. (2014) 7,8-dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis; Neurosci. Lett., 566 286
Properties of 7,8-Dihydroxyflavone
| Melting point: | 243-246°C |
| Boiling point: | 494.4±45.0 °C(Predicted) |
| Density | 1.443±0.06 g/cm3(Predicted) |
| FEMA | 4830 | 7,8-DIHYDROXYFLAVONE |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO: soluble24mg/mL |
| form | solid |
| appearance | yellow powder |
| pka | 6.86±0.40(Predicted) |
| color | Yellow or tan |
| Stability: | Stable for 1 year from date of purchase as supplied. Protect from moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 week. |
| CAS DataBase Reference | 38183-03-8(CAS DataBase Reference) |
Safety information for 7,8-Dihydroxyflavone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 7,8-Dihydroxyflavone
| InChIKey | COCYGNDCWFKTMF-UHFFFAOYSA-N |
7,8-Dihydroxyflavone manufacturer
OCIMUM LIFESCIENCES PVT LTD
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

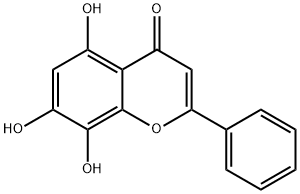


You may like
-
 7,8-Dihydroxyflavone CAS 38183-03-8View Details
7,8-Dihydroxyflavone CAS 38183-03-8View Details
38183-03-8 -
 TrkB Agonist, 7,8-Dihydroxyflavone CAS 38183-03-8View Details
TrkB Agonist, 7,8-Dihydroxyflavone CAS 38183-03-8View Details
38183-03-8 -
 7,8-Dihydroxyflavone hydrate CAS 38183-03-8View Details
7,8-Dihydroxyflavone hydrate CAS 38183-03-8View Details
38183-03-8 -
 38183-03-8 98%View Details
38183-03-8 98%View Details
38183-03-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
