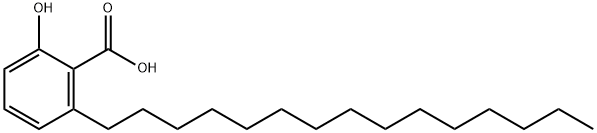
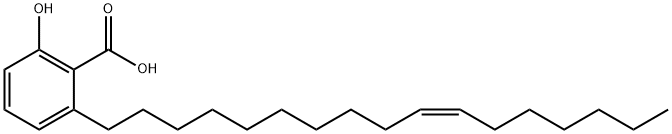
6-pentadecylsalicylic acid
Synonym(s):22:0-Anacardic acid;2-Hydroxy-6-pentadecylbenzoic acid;6-Pentadecylsalicylic acid;Anacardic acid;
- CAS NO.:16611-84-0
- Empirical Formula: C22H36O3
- Molecular Weight: 348.52
- MDL number: MFCD07368254
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
What is 6-pentadecylsalicylic acid?
Description
Anacardic acid, isolated from cashew shells or several other medicinal plants, is the general name given to a family of four different 6-
The Uses of 6-pentadecylsalicylic acid
Anacardic acid, isolated from cashew shells or several other medicinal plants, is the general name given to a family of four different 6-alkyl salicyclic acids having varying degrees of unsaturation in the 15-carbon alkyl chain. These compounds are associated with anti-inflammatory, anti-tumor, molluscicidal, and anti-microbial activity. Literature frequently sites and gives the name anacardic acid to the completely-saturated compound (6-pentadecyl salicylic acid). Anacardic acid inhibits the histone acetyltransferase (HAT) activity of the transcription co-activators p300 and p300/CREB-binding protein-associated factor (PCAF) with IC50 values of 8.5 and 5 μM, respectively. At 25 μmol/L, anacardic acid suppresses NF-κB activation, inhibits IκB-α phosphorylation, and prohibits p65 nuclear translocation in KBM-5 cells.[Cayman Chemical]
The Uses of 6-pentadecylsalicylic acid
2-Hydroxy-6-pentadecyl Benzoic Acid, is a compound that is used to treat agriculture from various fruit insects. Derivative of this molecule has been also shown to be a potent activator of human 5-lipoxygenases (5-LOX).
The Uses of 6-pentadecylsalicylic acid
Anacardic acid has been used:
- as a histone acetylase (HAT) inhibitor to study its effects on rat cortical neurons
- as a positive control in acetylation assay in vitro
- as an acetylase inhibitor to study its effects on the ribonucleic acid export 1 (Rae-1) protein acetylation that was transfected in human embryonic kidney cells
What are the applications of Application
Anacardic Acid is shown to potently inhibit HAT-dependent transcription and SUMOylation
Definition
ChEBI: Anacardic acid is a hydroxybenzoic acid that is salicylic acid substituted by a pentadecyl group at position 6. It is a major component of cashew nut shell liquid and exhibits an extensive range of bioactivities. It has a role as an EC 2.3.1.48 (histone acetyltransferase) inhibitor, an apoptosis inducer, a neuroprotective agent, an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor, an anticoronaviral agent, an antibacterial agent, an anti-inflammatory agent and a plant metabolite. It is a hydroxybenzoic acid and a hydroxy monocarboxylic acid. It is functionally related to a salicylic acid.
General Description
A cell-permeable ginkgolic acid (Cat. No. 345887) analog that inhibits protein SUMO (Cat. Nos. 662037, 662039, and 662042) modification (IC50 = 2.2 μM using RanGAP1-C2 as substrate) in an ATP-dependent manner by selectively targeting SUMO-activating enzyme E1 (Cat. Nos. 662073 and 662074) and interfering with E1-SUMO intermediate formation. Both anacardic acid and ginkgolic acid are shown to effectively decrease overall SUMOylation of 293T cellular proteins in a dose-dependent manner, while neither compound is effective in affecting overall cellular protein ubiquitination or histone H4K8 acetylation in 293T cultures, although anacardic acid is shown to inhibit p300 (Cat. No. 506200) and PCAF (Cat. No. 124026) histone acetyltransferase activities in cell-free acetylase assays (by 82% and 86%, respectively, at 10 μM). Also reported to inhibit the activity of prostaglandin synthase, tyrosinase, and lipoxygenase, as well as to enhance Aurora kinase A (Cat. No. 481413), but not Aurora kinase B, autophosphorylation and kinase activity by inducing conformation change and enhancing ATP binding.
Biochem/physiol Actions
Target IC50: 5 μM against HAT; 8.5 μM against PCAF; 2.2 μM inhibiting protein SUMO modification using RanGAP1-C2 as substrate
in vitro
lncap, a classical metastatic prostate adenocarcinoma cell line, was adopted to study the effect of aa on cell growth, cycles and apoptosis. it was found that 125 m aa significantly inhibited lncap cell proliferation. in addition, the g1/s cell cycles arrest and the apoptosis of lncap cell was induced. further mechanistic study suggested that aa induced cell apoptosis via suppressing p300. [1]
in vivo
diesel exhaust particle- (dep-) induced lung inflammation model was established to study the effect of aa on inflammation in mice. ten days before dep-instillation stimulation, mice were orally pretreated with 50, 150, or 250 mg/kg of aa for thirty days. all doses of aa ameliorated activities of oxidative enzymes. moreover, 50 mg/kg of aa significantly decreased the expression level of tumor necrosis factor in lung. [2]
storage
-20°C
References
1) Balasubramanyam et al. (2003), Small molecule modulators of histone acetyltransferase p300; J. Biol. Chem. 278 19134 2) Sun et al. (2006), Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation; FEBS Lett. 580 4353 3) Wu et al. (2011), Anacardic acid (6-Pentadecylsalicylic acid) Inhibits Tumor Angiogenesis by Targeting Src/FAK/Rho GTPases Signaling Pathway; J. Pharmacol. Exp. Ther. 339 403 4) Omanakuttan et al. (2012), Anacardic acid Inhibits the Catalytic Activity of Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9; Mol. Pharmacol. 82 614 5) Sung et al. (2008), Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kB- regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kBα kinase, leading to potentiation of apoptosis; Blood 111 4880
Properties of 6-pentadecylsalicylic acid
| Melting point: | 90-91℃ |
| Boiling point: | 474.8±33.0 °C(Predicted) |
| Density | 1.002 |
| storage temp. | -20°C |
| solubility | DMSO: ≥20mg/mL |
| pka | 3.08±0.30(Predicted) |
| form | White solid |
| color | white to beige |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
Safety information for 6-pentadecylsalicylic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 6-pentadecylsalicylic acid
| InChIKey | ADFWQBGTDJIESE-UHFFFAOYSA-N |
New Products
ALUMINIUM IODIDE 100 GM BUFFER CAPSULE PH 7.0 - 10 CAP BUFFER SOLUTION PH 9.5 (BORATE) EZEE BLUE GEL STAINER BORAX CARMINE (GRENACHERS ALCOHOLIC) POTASSIUM IODATE - IODIDE SOLN 0.1 N Dabigatran Acyl-O3-D-Glucuronide Trifluoroacetic Acid Salt Isofolic Acid Dabigatran 2-O-acylglucuronide metabolite Dabigatran Acyl-?-D- glucuronide Trifluroacetic Acid Erythromycin EP Impurity A Desloratidine Related Compound ARelated products of tetrahydrofuran
You may like
-
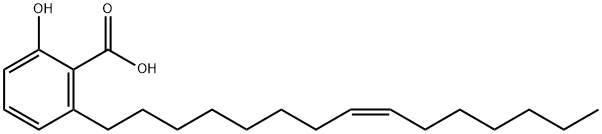
 Anacardic Acid CAS 16611-84-0View Details
Anacardic Acid CAS 16611-84-0View Details
16611-84-0 -
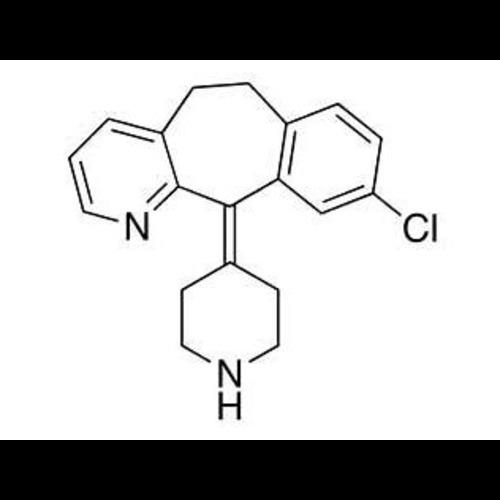 Dechloro DesloratadineView Details
Dechloro DesloratadineView Details -
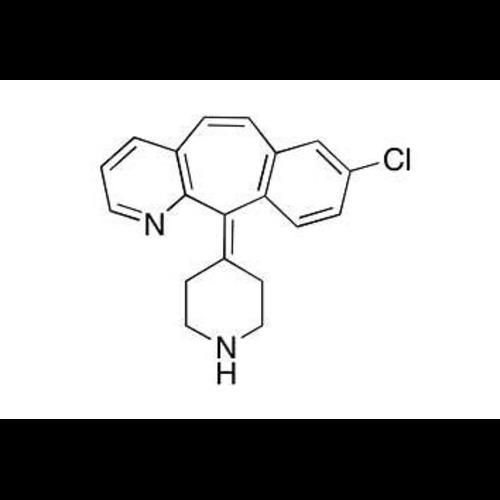 Dehydro DesloratadineView Details
Dehydro DesloratadineView Details -
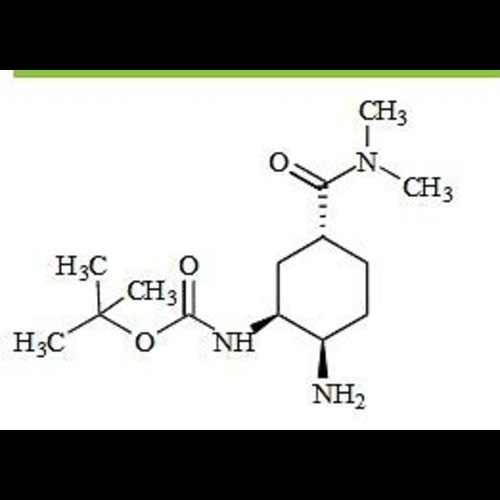 Edoxaban Impurity 57View Details
Edoxaban Impurity 57View Details
2089454-69-1 -
 Eltrombopag N-Oxide ImpurityView Details
Eltrombopag N-Oxide ImpurityView Details
2734533-17-4 -
 Empagliflozin Bromo ImpurityView Details
Empagliflozin Bromo ImpurityView Details -
 Glycopyrronium Bromide EP Impurity IView Details
Glycopyrronium Bromide EP Impurity IView Details
1404617-94-2 -
 Ipratropium EP Impurity BView Details
Ipratropium EP Impurity BView Details
58073-59-9