5-HYDROXYINDOLE-3-ACETIC ACID
- CAS NO.:54-16-0
- Empirical Formula: C10H9NO3
- Molecular Weight: 191.18
- MDL number: MFCD00005639
- EINECS: 200-195-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
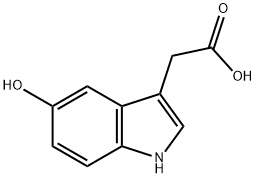
What is 5-HYDROXYINDOLE-3-ACETIC ACID?
Description
5-hydroxy Indole-3-acetic acid (5-HIAA) is the primary metabolite of serotonin (5-HT; ). It is formed when 5-HT is metabolized by monamine oxidase and aldehyde dehydrogenase in the liver. 5-HIAA is excreted in urine and has been used as a biomarker for detection of neuroendocrine tumors and an internal standard for the quantification of serotonin metabolism in rat brain using mass spectrometry. 5-HIAA has also been associated with autism, insomnia, and chronic migraine.
Chemical properties
beige crystalline powder
The Uses of 5-HYDROXYINDOLE-3-ACETIC ACID
5-Hydroxyindole-3-acetic acid is a major serotonin metabolite.
The Uses of 5-HYDROXYINDOLE-3-ACETIC ACID
5-Hydroxyindole-3-acetic acid is an impurity of chlorpheniramine (C424300), an antihistaminic agent.
Definition
ChEBI: A member of the class of indole-3-acetic acids that is indole-3-acetic acid substituted by a hydroxy group at C-5.
What are the applications of Application
5-Hydroxyindole-3-acetic acid is a major serotonin metabolite
General Description
5-Hydroxyindole-3-acetic acid (5-HIAA) is the catabolic end product of serotonin pathway. 5-HIAA is present in body fluids like cerebrospinal fluid (CSF), blood and urine. The expression levels of 5-HIAA is different among the normal and cancer patients. Sepiapterin reductase deficiency results in low level of 5-HIAA in CSF. 5-HIAA level is low in CSF in patients with bipolar 1 disorder with childhood attention-deficit hyperactivity disorder (ADHD).
Mechanism of action
The primary product of the metabolism of serotonin. Formed by the oxidative deamination of serotonin by monoamine oxidase, presumably the A isozyme, 5-HIAA is subsequently cleared by an active transport mechanism. Unlike serotonin, 5-HIAA is not a neurotransmitter and thus its formation contributes to the termination of the synaptic actions of serotonin. Inhibition of monoamine oxidase activity enhances and prolongs the actions of serotonin.
Properties of 5-HYDROXYINDOLE-3-ACETIC ACID
| Melting point: | 161-164 °C (dec.) (lit.) |
| Boiling point: | 326.92°C (rough estimate) |
| Density | 1.2722 (rough estimate) |
| refractive index | 1.5310 (estimate) |
| storage temp. | -20°C |
| solubility | Soluble in ethanol (50 mg/ml). |
| pka | 4.54±0.30(Predicted) |
| form | crystalline |
| color | off-white to purple |
| Sensitive | Light Sensitive |
| BRN | 168797 |
| Stability: | Air Sensitive, Light Sensitive |
| CAS DataBase Reference | 54-16-0 |
Safety information for 5-HYDROXYINDOLE-3-ACETIC ACID
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 5-HYDROXYINDOLE-3-ACETIC ACID
| InChIKey | DUUGKQCEGZLZNO-UHFFFAOYSA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![N-[(tert-Butoxy)carbonyl]-D-tryptophan](https://img.chemicalbook.in/CAS/GIF/5241-64-5.gif)
You may like
-
 5-Hydroxyindole-3-acetic Acid CAS 54-16-0View Details
5-Hydroxyindole-3-acetic Acid CAS 54-16-0View Details
54-16-0 -
 5-Hydroxyindole-3-acetic acid CAS 54-16-0View Details
5-Hydroxyindole-3-acetic acid CAS 54-16-0View Details
54-16-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1