1-Methylindole-3-carboxaldehyde
Synonym(s):3-Formyl-1-methylindole;NSC 83042
- CAS NO.:19012-03-4
- Empirical Formula: C10H9NO
- Molecular Weight: 159.18
- MDL number: MFCD00014570
- EINECS: 242-750-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-01-29 17:42:43
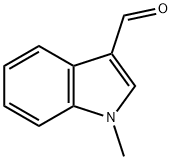
What is 1-Methylindole-3-carboxaldehyde?
Chemical properties
light yellow to orange or brown crystalline powder
The Uses of 1-Methylindole-3-carboxaldehyde
1-Methylindole-3-carboxaldehyde is used as a reactant for preparation of nitroolefins and β-nitroalcohols via microwave- or ultrasound-assisted Henry reactions, reactant for synthesis of quinolinones via three-component Ugi reaction, reactant for synthesis of α-ketoamides as inhibitors of Dengue virus protease with antiviral activity in cell-culture, reactant for preparation of thiazolopyrimidinones as inhibitors of Bcl-2 proteins, reactant for preparation of vinylindoles via Peterson olefination or olefination with Nysted reagent, reactant for preparation of indolyl alkenes from microwave-enhanced Knoevenagel condensation as antibacterial agents 1-Methylindole-3-carboxaldehyde may be used in the synthesis of (Z)-3-(1-methyl-1H-indol-3-yl)-2-(thiophen-3-yl)acrylonitrile, via base-catalyzed condensation with thiophene-3-acetonitrile. It was also used in the preparation of monomer, required for the synthesis of poly(3-vinyl-1-methylindole).
The Uses of 1-Methylindole-3-carboxaldehyde
Reactant for preparation of nitroolefins and β-nitroalcohols via microwave- or ultrasound-assisted Henry reactions Reactant for synthesis of quinolinones via three-component Ugi reaction Reactant for synthesis of α-ketoamides as inhibitors of Dengue virus protease with antiviral activity in cell-culture Reactant for preparation of thiazolopyrimidinones as inhibitors of Bcl-2 proteins Reactant for preparation of vinylindoles via Peterson olefination or olefination with Nysted reagent Reactant for preparation of indolyl alkenes from microwave-enhanced Knoevenagel condensation as antibacterial agents.
Synthesis Reference(s)
Tetrahedron, 49, p. 4015, 1993 DOI: 10.1016/S0040-4020(01)89915-4
Synthesis, p. 396, 1987 DOI: 10.1055/s-1987-27960
General Description
1-Methylindole-3-carboxaldehyde is a heterocyclic indole aldehyde. 1-Methylindole-3-carboxaldehyde on condensation with 2-hydroxybenzohydrazide yields Schiff base.
Properties of 1-Methylindole-3-carboxaldehyde
| Melting point: | 70-72 °C (lit.) |
| Boiling point: | 186-189 °C(Press: 3-4 Torr) |
| Density | 1.10±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| form | Crystalline Powder and Chunks |
| color | Light yellow to orange or brown |
| Water Solubility | Insoluble in water. |
| Sensitive | Air Sensitive |
| BRN | 121302 |
| CAS DataBase Reference | 19012-03-4(CAS DataBase Reference) |
Safety information for 1-Methylindole-3-carboxaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1-Methylindole-3-carboxaldehyde
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 19012-03-4 1-Methylindole-3-carboxaldehyde-98% 99%View Details
19012-03-4 1-Methylindole-3-carboxaldehyde-98% 99%View Details
19012-03-4 -
 1-Methyl-3-formylindole CAS 19012-03-4View Details
1-Methyl-3-formylindole CAS 19012-03-4View Details
19012-03-4 -
 1-methylindole-3-carboxaldehyde CAS 19012-03-4View Details
1-methylindole-3-carboxaldehyde CAS 19012-03-4View Details
19012-03-4 -
 1-Methylindole-3-carboxaldehyde CAS 19012-03-4View Details
1-Methylindole-3-carboxaldehyde CAS 19012-03-4View Details
19012-03-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8