5'-DEOXYTHYMIDINE
- CAS NO.:3458-14-8
- Empirical Formula: C10H14N2O4
- Molecular Weight: 226.23
- MDL number: MFCD00057398
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
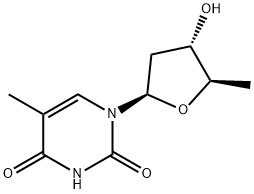
What is 5'-DEOXYTHYMIDINE?
The Uses of 5'-DEOXYTHYMIDINE
The pyrimidine thymidine consists of thymine combined with deoxyribose. Prior to polymerization into DNA, thymidine must be phosphorylated at the hydroxyl group on carbon 5 of the ribose moiety. 5'-Deoxythymidine is a form of thymidine in which the hydroxyl group on carbon 5 of ribose has been replaced with hydrogen. As a result, this compound cannot be phosphorylated and used by DNA polymerase in the synthesis of DNA. 5'-Deoxythymidine is readily imported by cellular nucleoside importers and competitively inhibits the influx of thymidine. It demonstrates antibacterial activity against B. subtilis and S. aureus.[Cayman Chemical]
What are the applications of Application
5′-Deoxythymidine is a cell synchronizing nucleoside
Biological Activity
5'-deoxy thymidine has been used as a research tool for antiviral and anticancer studies. 5'-deoxy thymidine can be used to study the entry mechanism into human erythrocytes in comparison with other deoxythymidines. deoxythymidine, also named as thymidine, is the dna nucleoside t. deoxythymidine can pair with deoxyadenosine (a) in double-stranded dna. 5'-deoxy thymidine is a form of thymidine which had the hydroxyl group at the 5' position replaced with hydrogen [1]. 5'-deoxy thymidine (50 μg/ml) was effective against bacillus subtilis and staphylococcus aureus [1]. the mbc of 5'-deoxy thymidine was 50 μg/ml. 5'-deoxy thymidine has been used as an antibacterial agent in medical situations [1].thymidine has been phosphorylated at the hydroxyl group on carbon 5 of the ribose moiety before polymerization into dna. 5'-deoxy thymidine cannot be phosphorylated and used by dna polymerase in the synthesis of dna. in human erythrocytes, 5'-deoxy thymidine was readily and nonconcentratively uptaked by metabolism, and could be partially inhibited by nucleosides or inhibitors of nucleoside transport at micromolar concentrations [2].
References
[1] hatano a, nishimura m, souta i. impact of unnatural nucleosides on the control of microbial growth[j]. biocontrol science, 2009, 14(2): 55-60.
[2] domin b a, mahony w b, koszalka g w, et al. membrane permeation characteristics of 5'-modified thymidine analogs[j]. molecular pharmacology, 1992, 41(5): 950-956.
Properties of 5'-DEOXYTHYMIDINE
| Melting point: | 192-193 °C(lit.) |
| Density | 1.344±0.06 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | ≤10mg/ml in DMSO;16mg/ml in dimethyl formamide |
| form | crystalline solid |
| pka | 9.55±0.10(Predicted) |
| color | Off-white to light yellow |
Safety information for 5'-DEOXYTHYMIDINE
Computed Descriptors for 5'-DEOXYTHYMIDINE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1