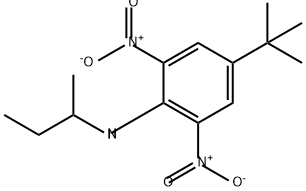
4,6-Dinitro-2-sec-butylphenol
Synonym(s):2-sec-Butyl-4,6-dinitrophenol
- CAS NO.:88-85-7
- Empirical Formula: C10H12N2O5
- Molecular Weight: 240.21
- MDL number: MFCD00024246
- EINECS: 201-861-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
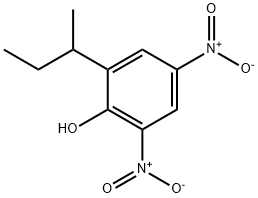
What is 4,6-Dinitro-2-sec-butylphenol?
Description
Dinoseb, also known as dinitrophenol, can adversely affect the
energy generating reaction in a cell. No cell will live very long
under the influence of high concentrations of dinitrophenol. It
makes the body burn enough energy to result in weight loss.
During the 1930s, physicians unwittingly prescribed certain
types of dinitrophenol as diet pills.
Dow Chemical changed the basic structure of dinitrophenol
slightly to produce dinoseb, which was marketed in 1948.
Dinoseb was widely used as a contact herbicide against
broadleaf weeds.
Dinoseb causes toxicity the same way in plants,
animals, and fungi because all cells contain very similar
biochemical pathways for creating energy from the breakdown
of sugars. Furthermore, photosynthesis in plants
relies on an energy transfer system that is also inhibited by
dinitrophenol.
Given the high toxicity, EPA concluded that the doses
causing the birth defects and the endocrine-disrupting effects
were close to worker exposure levels. Thus, under an emergency
order issued in 1986, EPA suspended dinoseb’s registration.
In August 1990, the EPA banned the burying of
dinoseb-contaminated soils in EPA-approved landfills,
making incineration the only EPA-approved disposal method
for dinoseb-contaminated soil. Incineration is expensive and
incomplete, leaving a noncombustible residue as a further
hazardous waste and some combustion products that could
remain toxic. Therefore, an ex situ soil bioremediation process
was developed by the Sabre Processing company. This process
is known as the SABRE process; it uses an anaerobic consortium
and supplemental carbon source at the field scale to
successfully remediate contaminated soils.
Chemical properties
solid
The Uses of 4,6-Dinitro-2-sec-butylphenol
The amine, ammonium salt or acetate ester is used as a contact herbicide for postemergence weed control in cereals, cotton, peas, beans, potatoes, pumpkins, soybeans and strawberries.
The Uses of 4,6-Dinitro-2-sec-butylphenol
Dinoseb is used as an herbicide andinsecticide.
The Uses of 4,6-Dinitro-2-sec-butylphenol
Dinoseb is used as an herbicide, corn yield enhancer, insecticide, and miticide. It is used as a herbicide in soybeans, a variety of vegetables, fruits, nuts, citrus trees, and with other field crops for control of grasses and broadleaf weeds. It is used as an insecticide in grapes.
Definition
ChEBI: 2-(butan-2-yl)-4,6-dinitrophenol is a dinitrophenol that is 2,4-dinitrophenol substituted by a butan-2-yl group at position 2.
General Description
Orange-brown viscous liquid or orange-brown solid. Orange crystals when pure. Has a pungent odor. Used as a plant growth regulator; insecticide and herbicide.
Reactivity Profile
4,6-Dinitro-2-sec-butylphenol is a powerful oxidizing agent. . 4,6-Dinitro-2-sec-butylphenol is dangerously explosive. When not water wet 4,6-Dinitro-2-sec-butylphenol is a high explosive. Dry, the material is easily ignited and 4,6-Dinitro-2-sec-butylphenol will burn very vigorously. On decomposition, nitro compounds such as this emit toxic fumes. Appear to be stable in acid solution, but are susceptible to decomposition by ultraviolet radiation in alkaline solution. [EPA, 1998].
Health Hazard
Extremely toxic: Probable oral lethal dose is 5-50 mg/kg; between 7 drops and 1 teaspoonful for 70 kg person (150 lb.).
Health Hazard
Dinoseb is a highly toxic compound. Theoral LD50 values in small laboratory animals were between 10 and 25 mg/kg. Acutetoxicity tests on daphnids and fathead minnows showed high toxicity. The LC50 values in both these species are 0.24 and0.17 mg/L, respectively (Gersich and Mayes1986). Pregnant white rabbits treated withdinoseb exhibited maternal toxicity above thedose level of 1 mg/kg/day. At highly toxicdose levels, adverse effects were observedin developing fetuses (Johnson et al. 1988).Oral administration of dinoseb producedtumors in lung and liver in mice.
Fire Hazard
This is a dinitrophenol herbicide. (Non-Specific -- Dinitrophenol, Flammable Solid). 4,6-Dinitro-2-sec-butylphenol is dangerously explosive. When not water wet 4,6-Dinitro-2-sec-butylphenol is a high explosive. Dry, the material is easily ignited and 4,6-Dinitro-2-sec-butylphenol will burn very vigorously. On decomposition, nitro compounds such as this emit toxic fumes. Appear to be stable in acid solution, but are susceptible to decomposition by ultraviolet radiation in alkaline solution.
Flammability and Explosibility
Non flammable
Agricultural Uses
Plant growth regulator, Herbicide: Dinoseb is a phenolic herbicide used in soybeans, vegetables, fruits and nuts, citrus, and other field crops for the selective control of grass and broadleaf weeds (e.g., in corn). It is also used as an insecticide in grapes, and as a seed crop drying agent. It is produced in emuslifiable concentrates or as water-soluble ammonium or amine salts. It is no longer available in the U.S. Formerly widely used in the UK for the fumigation of potatoes; however, dinoseb acetate and dinoseb amine were banned from use in 1988. Dinoseb’s primary use is as a contact herbicide for post-emergence weed control in cereals, undersown cereals, seedling lucerne and peas. Dinoseb is also used as a corn yield enhancer and an insecticide and miticide. Banned for use in EU countries (includes salts and acetate). A U.S. EPA restricted Use Pesticide (RUP). The use of dinoseb was canceled in the U.S. in 1986 based on the potential risk of birth defects and other adverse health effects for applicators and other persons having substantial dinoseb exposure. There are 20 global suppliers.
Trade name
AATOX®; AI3-01122®; ARETIT®; BASANITE®; BNP 20®; BNP 30®; BUTAPHENE®; CALDON®; CASWELL No. 392DD®; CHEMOX®[C]; CHEMOX GENERAL®[C]; CHEMOX P. E. ®[C]; CHEMSECT DNBP®; DESICOIL®; DIBUTOX®; DINITRALL®; DINITRO®; DN 289®; DOW GENERAL®[C]; DOW GENERAL WEED KILLER®[C]; DOW SELECTIVE WEED KILLER®[C]; DYNAMYTE®[C]; DYTOP®; ELGETOL 318®; FANICIDE®; GEBUTOX®; HEL-FIRE®[C]; HIVERTOX®; HOE 26150®; IVOSIT®; KILOSEB®; KNOWX-WEED®; KNOX-WEED®; LADOB®; LASEB®; LIRO DNBP®; NITROPONE C®; PERSEVTOX®; PHENOTAN®; PREMERGE®; SINOX GENERAL®[C]; SPARIC®; SPURGE®; SUBITEX®; UNICROP DNBP®; VERTAC DINITRO WEED KILLER®[C]; VERTAC GENERAL WEED KILLER®[C]; VERTAC SELECTIVE WEED KILLER®[C]
Potential Exposure
AgriculturalChemical; Tumorigen, Mutagen; Reproductive Effector;Primary Irritant. This material is used for herbicide formulation; as a plant growth regulator, insecticide, and herbicide. Dinoseb is primarily used as a contact herbicide forpostemergence weed control in cereals, undersown cereals,seedling lucerne and peas. Dinoseb is also used as a cornyield enhancer and an insecticide and miticide.[US EPA]
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for atleast 15 min, occasionally lifting upper and lower lids.Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and washimmediately with soap and water. Seek medical attentionimmediately. If this chemical has been inhaled, removefrom exposure, begin rescue breathing (using universalprecautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give largequantities of water and induce vomiting. Do not make anunconscious person vomit. Consult poison center on useof antidotes
Environmental Fate
Biological. When 14C-labeled dinoseb (5 ppm) was incubated in soil at 25°C for 60 days, 36.0% of the applied amount degraded to 14CO2 (Doyle et al., 1978). Thom and Agg (1975) reported that dinoseb is unlikely to be degraded in conventional sewage treatment processes.
Groundwater. According to the U.S. EPA (1986) dinoseb has a high potential to leach to groundwater.
Plant. When dinoseb on bean leaves was exposed to sunlight, photodegradation resulted in the formation of persistent, polar compounds. The compounds could not be identified by TLC (Matsuo and Casida, 1970).
Chemical/Physical. Reacts with organic and inorganic bases forming water-soluble salts (Worthing and Hance, 1991).
Emits toxic fumes of chlorine when heated to decomposition (Sax and Lewis, 1987).
storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with dinoseb youshould be trained on its proper handling and storage. Store intightly closed containers in a cool, well-ventilated area
Shipping
Dinoseb and dinoseb acetate are toxic pesticideand should be labeled “SUBSTITUTED NITROPHENOLPESTICIDES, SOLID (or LIQUID), TOXIC.” They fallinto DOT/UN Hazard Class 6.1(b) and Packing Group III.
Toxicity evaluation
Dinoseb does not ordinarily persist in the soil, but in storage areas or locations where it has been spilled, it persists as a soil and groundwater contaminant. Additionally, release of dinoseb may result primarily from its use as an herbicide on a variety of weeds. Release of dinoseb to soil is expected to result in biodegradation, and dinoseb only weakly adsorbs to soils and therefore leaches to groundwater. However, it may bind more strongly to clay soils, especially at acidic pH. Photolytic degradation of dinoseb from soil surface may be important. Volatilization is not expected to be significant. In the absence of volatilization, the half-life of dinoseb in the sandy loam soil was estimated to be about 100 days. Dinoseb may photodegrade in surface water with a half-life of 14–18 days. Hydrolysis in water may not be important. It is unlikely to undergo significant biodegradation in most natural waters. Volatilization from water is expected to be slow and bioconcentration is expected to be insignificant. Based on its vapor pressure of 8.5 × 10-2mmHg at 20 ℃, dinoseb may exist entirely in the vapor phase in the atmosphere. The half-life for the reaction of vapor phase dinoseb with photochemically generated hydroxyl radicals in the atmosphere was estimated to be 14.1 days. Wet deposition may remove some of the compound from air.
Incompatibilities
The solution in water is a weak acid.Attacks many metals in the presence of water.
Properties of 4,6-Dinitro-2-sec-butylphenol
| Melting point: | 55.5°C |
| Boiling point: | 382.92°C (rough estimate) |
| Density | 1.29 |
| vapor pressure | 0.01Pa at 25℃ |
| refractive index | 1.6620 (estimate) |
| Flash point: | >100 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 4.62(at 25℃) |
| Specific Gravity | 1 |
| Water Solubility | 0.0052 g/100 mL |
| Merck | 13,3317 |
| Stability: | Stable. Strong oxidizing agent. Incompatible with reducing agents, combustible material. |
| CAS DataBase Reference | 88-85-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Sec-butyl-4,6-dinitrophenol(88-85-7) |
| EPA Substance Registry System | Dinoseb (88-85-7) |
Safety information for 4,6-Dinitro-2-sec-butylphenol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H311:Acute toxicity,dermal H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H360:Reproductive toxicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4,6-Dinitro-2-sec-butylphenol
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1