tert-Butylhydroquinone
Synonym(s):2-(1,1-Dimethylethyl)-1,4-benzenediol;2-tert.-Butyl-1,4-dihydroxybenzene;NSC 4972;tert-Butylhydroquinone
- CAS NO.:1948-33-0
- Empirical Formula: C10H14O2
- Molecular Weight: 166.22
- MDL number: MFCD00002344
- EINECS: 217-752-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
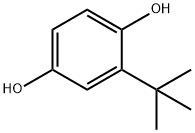
What is tert-Butylhydroquinone?
Chemical properties
White, crystalline solid having a characteristic odour.
Tert-Butylhydroquinone (TBHQ) is a versatile antioxidant with a range of applications. It is commonly used to preserve oils, fats, and food items, and can be found in vegetable oils, animal fats, varnishes, lacquers, resins, oil field additives, and perfumes. TBHQ demonstrates cytoprotective qualities at low concentrations, but it becomes cytotoxic at higher concentrations. Its antimicrobial properties have been studied in the context of inactivating barotolerant strains of Listeria monocytogenes and Escherichia coli. Additionally, TBHQ has been utilized in the development of environmentally friendly electrode materials for supercapacitors, where it is used to decorate the surface of graphene nanosheets.
The Uses of tert-Butylhydroquinone
tert-Butylhydroquinone (TBHQ) is an antioxidant that exhibits an excellent stabilizing effect in unsaturated fats and oils. It has good solubility in fats and oils, with a maximum usage level of 0.02% based on the weight of the fat or oil or the fat content of the food product. It shows no discoloration in the presence of iron and produces no discernible flavor or odor. It can be combined with BHA and BHT. It is used in edible fats and vegetable oils to retard rancidity. It is used in potato chips and dry cereal. It is also termed butylhydroquinone and mono-tertiary-butylhydroquinone.
The Uses of tert-Butylhydroquinone
antioxidant in cosmetic products like lipsticks.
The Uses of tert-Butylhydroquinone
TBHQ was used to study the inactivation of barotolerant strains of Listeria monocytogenes and Escherichia coli. Environment friendly electrode materials for supercapacitors were attained by decorating the surface of graphene nanosheets with TBHQ. TBHQ is frequently used as a food preservative. Inhibition of oxidative rancidity in frozen cooked fish flakes by tert-butylhydroquinone. Nuclear factor E2-related factor 2-dependent antioxidant response element activation is by tert-butylhydroquinoneoccurring preferentially in Astrocytes conditions.
What are the applications of Application
tert-Butylhydroquinone (TBHQ) is a phenolic antioxidant. TBHQ is frequently used as a food preservative. In low concentrations it shows cytoprotective qualities while at higher concentrations it exhibits cytotoxic behavior.
Definition
ChEBI: A member of the class of hydroquinones in which one of the ring hydrogens of hydroquinone is replaced by a tert-butyl group.
General Description
White to light tan crystalline powder or a fine beige powder. Very slight aromatic odor.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phenols, such as tert-Butylhydroquinone, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. Nitrated phenols often explode when heated. Many of them form metal salts that tend toward detonation by rather mild shock. tert-Butylhydroquinone is incompatible with oxidizers.
Fire Hazard
tert-Butylhydroquinone is combustible.
Flammability and Explosibility
Non flammable
Contact allergens
This antioxidant has seldom been reported as a sensitizer, mainly in cosmetics (lipsticks, lip-gloss, hair dyes) or in cutting oils. Simultaneous/cross-reactions have been described to butylhydroxyanisole (BHA) and less frequently to butylhydroxytoluene (BHT), but not to hydroquinone
Description
TBHQ was used to study the inactivation of barotolerant strains of Listeria monocytogenes and Escherichia coli. Environment friendly electrode materials for supercapacitors were attained by decorating the surface of graphene nanosheets with TBHQ.
Purification Methods
Recrystallise the hydroquinone from H2O or MeOH and dry it in a vacuum at 70o. Store it in a dark container. [Stroh et al. Angew Chem 69 699 1957, Beilstein 6 IV 6013.]
Properties of tert-Butylhydroquinone
| Melting point: | 127-129 °C(lit.) |
| Boiling point: | 295 °C |
| Density | 295 |
| vapor pressure | 0.004Pa at 25℃ |
| refractive index | 1.4859 (estimate) |
| Flash point: | 171 °C |
| storage temp. | Store below +30°C. |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 10.80±0.18(Predicted) |
| form | Crystalline Powder |
| color | White to light tan, may contain black specs |
| Water Solubility | Slightly soluble in water(10g/L). |
| BRN | 637923 |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 1948-33-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,4-Benzenediol, 2-(1,1-dimethylethyl)-(1948-33-0) |
| EPA Substance Registry System | tert-Butylhydroquinone (1948-33-0) |
Safety information for tert-Butylhydroquinone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for tert-Butylhydroquinone
| InChIKey | BGNXCDMCOKJUMV-UHFFFAOYSA-N |
tert-Butylhydroquinone manufacturer
Tricon Exim
ASM Organics
Bazayan & Co.
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Tertiary Butyl Hydroquinone (TBHQ) 98%View Details
Tertiary Butyl Hydroquinone (TBHQ) 98%View Details -
 Tert-Butylhydroquinone 98%View Details
Tert-Butylhydroquinone 98%View Details -
 Tert-Butylhydroquinone CASView Details
Tert-Butylhydroquinone CASView Details -
 Powder Tert ButylhydroquinoneView Details
Powder Tert ButylhydroquinoneView Details
1948-33-0 -
 Tert-Butylhydroquinone, C10H14O2, CAS 1948-33-0, 98%, For Industrial UseView Details
Tert-Butylhydroquinone, C10H14O2, CAS 1948-33-0, 98%, For Industrial UseView Details
1948-33-0 -
 Tert Butyl Hydro QuinoneView Details
Tert Butyl Hydro QuinoneView Details
1948-33-0 -
 Powder Tertiary Butyl Hydroquinone, 98%, Grade Standard: Technical GradeView Details
Powder Tertiary Butyl Hydroquinone, 98%, Grade Standard: Technical GradeView Details
1948-33-0 -
 TBHQ Powder In ChennaiView Details
TBHQ Powder In ChennaiView Details
1948-33-0
