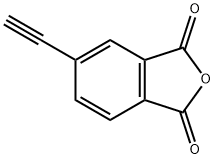
4-PHENYLETHYNYLPHTHALIC ANHYDRIDE
- CAS NO.:119389-05-8
- Empirical Formula: C16H8O3
- Molecular Weight: 248.23
- MDL number: MFCD10574892
- EINECS: 601-604-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:11:54
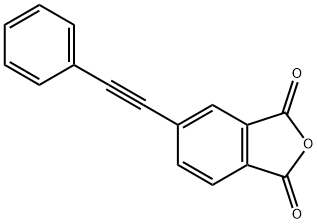
What is 4-PHENYLETHYNYLPHTHALIC ANHYDRIDE?
Description
4-Phenylethynylphthalic anhydride (PEPA) is a phthalic anhydride derivative with a phenylethynyl substituent in the 4-position.PEPA is commonly used as a reactive capping agent for polyimide synthesis and aromatic oligo-polyimides. Fluorinated aromatic oligoimides terminated with a phenylethynyl substituent melt completely in the range of 250-350 °C to form a low viscosity liquid. The melt viscosity of oligoimides increases with increasing molecular weight. After thermal curing at 371 °C, the thermoset polyimide resins produced show good thermal and mechanical properties. They showed a high glass transition temperature (Tg) of 300 °C, a flexural strength of 135.5 MPa, a flexural modulus of 3.1 GPa, a tensile strength of 65.7 MPa, and an elongation at break of 3.9%.
Chemical properties
Light yellow to light yellow-green crystal or powder
Preparation
The preparation of 4-phenylethynylphthalic anhydride is as follows:4-phenyl ethynyl dimethyl phthalate (33.6 g) was suspended in a mixed medium comprising water and methanol and a 25% by mass aqueous solution of sodium hydroxide (40 g) was dropwise added thereto with stirring. The resultant reaction mixture was stirred at 60° C. for 3 hours and, then, after confirming the completion of the reaction, cooled to the inside temperature of 30° C. Thereafter, 1 g of active carbon was added thereto, and, then, stirred for 30 minutes maintaining the same temperature. The resultant mixture was filtered to remove the active carbon, and rinsed with water. The filtrate and a rinsing solution were combined and, then, toluene and ethyl acetate were added to the resultant mixture. Concentrated hydrochloric acid (28 g) was dropwise added to the resultant 2-layered reaction mixture. The mixture was stirred for 30 minutes at room temperature and was allowed to stand, so that an organic layer containing 4-phenyl ethynyl phthalic acid was separated. After partially concentrating the organic layer, acetic anhydride (17 g) was added thereto, and the reaction mixture was refluxed with heating for 4 hours. After the reaction was completed, the resultant reaction mixture was cooled, so that 4-phenyl ethynyl phthalic anhydride was precipitated as a crystal. The crystal was filtered, rinsed and dried, to thereby obtain 26.6 g of 4-phenyl ethynyl phthalic anhydride as a pale yellow crystal. The yield was 94% on the basis of 4-phenyl ethynyl dimethyl phthalate.

Properties of 4-PHENYLETHYNYLPHTHALIC ANHYDRIDE
| Melting point: | 152 °C |
| Boiling point: | 466.9±28.0 °C(Predicted) |
| Density | 1.38±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| Water Solubility | Practically insoluble in water |
| form | powder to crystal |
| color | White to Yellow to Green |
| InChI | InChI=1S/C16H8O3/c17-15-13-9-8-12(10-14(13)16(18)19-15)7-6-11-4-2-1-3-5-11/h1-5,8-10H |
| EPA Substance Registry System | 1,3-Isobenzofurandione, 5-(phenylethynyl)- (119389-05-8) |
Safety information for 4-PHENYLETHYNYLPHTHALIC ANHYDRIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 4-PHENYLETHYNYLPHTHALIC ANHYDRIDE
| InChIKey | UPGRRPUXXWPEMV-UHFFFAOYSA-N |
| SMILES | C1(=O)C2=C(C=C(C#CC3=CC=CC=C3)C=C2)C(=O)O1 |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 4-Phenylethynylphthalic Anhydride CAS 119389-05-8View Details
4-Phenylethynylphthalic Anhydride CAS 119389-05-8View Details
119389-05-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
