4-Nitrophenylhydrazine
- CAS NO.:100-16-3
- Empirical Formula: C6H7N3O2
- Molecular Weight: 153.14
- MDL number: MFCD00007579
- EINECS: 202-824-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
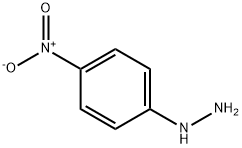
What is 4-Nitrophenylhydrazine?
Chemical properties
white to light yellow crystal powde
The Uses of 4-Nitrophenylhydrazine
Analytical reagent (aldehydes, ketones).
The Uses of 4-Nitrophenylhydrazine
4-Nitrophenylhydrazine is a nitro substituted aryl hydrazine with potential anticancer activity. Irritant.
Definition
ChEBI: A member of the class of phenylhydrazines that is phenylhydrazine substituted at the 4-position by a nitro group.
General Description
4-Nitrophenylhydrazine is a orange-red leaflet or needles wetted with water. 4-Nitrophenylhydrazine will emit toxic oxides of nitrogen when heated to decomposition. 4-Nitrophenylhydrazine is slightly heavier than water and insoluble in water. 4-Nitrophenylhydrazine may be irritating to skin, eyes and mucous membranes. 4-Nitrophenylhydrazine may be toxic by ingestion. 4-Nitrophenylhydrazine may be explosive and sensitive to friction in the dry state. 4-Nitrophenylhydrazine is used as a reagent for ketones and aliphatic aldehydes.
Reactivity Profile
4-NITROPHENYLHYDRAZINE is a powerful explosive. 4-Nitrophenylhydrazine can SELF-IGNITE in the presence of contaminants. Auto-ignition temperature may vary for 4-nitrophenylhydrazine similar to that of hydrazine which has an autoignition of as low as 74oC in contact with iron rust. Dangerous fire hazard or severe explosion can occur when exposed to heat, flame or oxidizing agents. Severe explosion may also occur by chemical reaction with alkali metals; BaO; CaO; K; Na; NH3; Cl2; chromates; CuO; Cu++ salts; F2; H2O2; iron rust; metallic oxides; PbO2;Ni; Ni(ClO4)2; HNO3; N2O; O2; liquid O2; K2Cr2O7; Na2Cr2O7; tetryl; zinc diamide; Zn(C2H5)2. Dangerous; when heated to decomposition 4-Nitrophenylhydrazine emits highly toxic nitrogen compounds; may explode by heat or chemical reaction. (Sax and Lewis, 1987) under Hydrazine p. 538-539.
Health Hazard
Some are toxic and may be fatal if inhaled, swallowed or absorbed through skin. Contact may cause burns to skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Safety Profile
Poison by intraperitoneal route.Mutation data reported. When heated to decomposition itemits toxic fumes of NOx. See also NITROCOMPOUNDS OF AROMATIC HYDROCARBONS
Purification Methods
Crystallise the hydrazine from EtOH. The hydrochloride has m 212o(dec) (from EtOH, also m 202-203o dec). [Beilstein 15 III 331, 15 IV 317.]
Properties of 4-Nitrophenylhydrazine
| Melting point: | 156 °C (dec.)(lit.) |
| Boiling point: | 276.04°C (rough estimate) |
| Density | 1.00 |
| refractive index | 1.6910 (estimate) |
| storage temp. | Explosives area |
| pka | 3.81±0.20(Predicted) |
| color | Orange-red leaflets or needles |
| Water Solubility | soluble in hot water |
| Merck | 14,6624 |
| BRN | 608107 |
| CAS DataBase Reference | 100-16-3(CAS DataBase Reference) |
| NIST Chemistry Reference | P-nitrophenyl hydrazine(100-16-3) |
| EPA Substance Registry System | Hydrazine, (4-nitrophenyl)- (100-16-3) |
Safety information for 4-Nitrophenylhydrazine
Computed Descriptors for 4-Nitrophenylhydrazine
4-Nitrophenylhydrazine manufacturer
Anand Agencies
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 4-Nitro phenyl hydrazine, GR 99%+ CAS 100-16-3View Details
4-Nitro phenyl hydrazine, GR 99%+ CAS 100-16-3View Details
100-16-3 -
 p-Nitrophenylhydrazine extrapure AR CAS 100-16-3View Details
p-Nitrophenylhydrazine extrapure AR CAS 100-16-3View Details
100-16-3 -
 4-NITROPHENYL HYDRAZINE AR CAS 100-16-3View Details
4-NITROPHENYL HYDRAZINE AR CAS 100-16-3View Details
100-16-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1