4-Methyl-1-pentene
- CAS NO.:691-37-2
- Empirical Formula: C6H12
- Molecular Weight: 84.16
- MDL number: MFCD00008949
- EINECS: 211-720-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
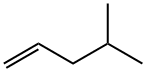
What is 4-Methyl-1-pentene?
Chemical properties
clear colourless liquid
The Uses of 4-Methyl-1-pentene
4-Methyl-1-pentene is used as a monomer for olefin polymerisation. The resulting polymer is poly(4-methyl-1-pentene). It can also be used to produce 1,2-diiodo-4-methyl-pentane.
The Uses of 4-Methyl-1-pentene
Organic synthesis, monomer for plastics used inautomobiles, electronic components, and laboratoryware.
General Description
Colorless liquid.
Air & Water Reactions
Highly flammable. Water insoluble.
Reactivity Profile
The unsaturated aliphatic hydrocarbons, such as 4-Methyl-1-pentene, are generally much more reactive than the alkanes. Strong oxidizers may react vigorously with them. Reducing agents can react exothermically to release gaseous hydrogen. In the presence of various catalysts (such as acids) or initiators, compounds in this class can undergo very exothermic addition polymerization reactions.
Hazard
Same as for 2-methyl-1-pentene.
Health Hazard
Harmful if inhaled or swallowed. Vapor or mist is irritating to the eyes, mucous membrane and upper respiratory tract. Causes skin irritation. Symptoms of exposure may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea and vomiting.
Fire Hazard
Special Hazards of Combustion Products: Vapors may travel considerable distance to source of ignition and flashback. Container explosion may occur under fire conditions. Forms explosive mixtures in air.
Source
California Phase II reformulated gasoline contained 4-methyl-1-pentene at a concentration of 300 mg/kg (Schauer et al., 2002).
Environmental Fate
Photolytic. Atkinson and Carter (1984) reported a rate constant of 1.06 x 10-16 cm3/molecule?sec
for the reaction of 4-methyl-1-pentene in the atmosphere.
Chemical/Physical. Complete combustion in air yields carbon dioxide and water.
Properties of 4-Methyl-1-pentene
| Melting point: | -155 °C |
| Boiling point: | 53-54 °C(lit.) |
| Density | 0.665 g/mL at 25 °C(lit.) |
| vapor density | >1 (vs air) |
| vapor pressure | 4.45 psi ( 20 °C) |
| refractive index | n |
| Flash point: | −25 °F |
| storage temp. | 0-6°C |
| solubility | Soluble in alcohol, benzene, chloroform, petroleum (Weast, 1986); miscible in pentene, hexane,
and heptene. |
| form | Liquid |
| color | Colorless |
| Water Solubility | Soluble in alcohol, benzene, chloroform, petroleum ether. Insoluble in water. |
| BRN | 1731096 |
| Henry's Law Constant | 0.615 atm?m3/mol at 25 °C (Hine and Mookerjee, 1975) |
| CAS DataBase Reference | 691-37-2(CAS DataBase Reference) |
| EPA Substance Registry System | 4-Methyl-1-pentene (691-37-2) |
Safety information for 4-Methyl-1-pentene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
Computed Descriptors for 4-Methyl-1-pentene
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 4-Methyl-1-pentene CAS 691-37-2View Details
4-Methyl-1-pentene CAS 691-37-2View Details
691-37-2 -
 4-Methyl-1-pentene CAS 691-37-2View Details
4-Methyl-1-pentene CAS 691-37-2View Details
691-37-2 -
 4-Methyl-1-pentene CAS 691-37-2View Details
4-Methyl-1-pentene CAS 691-37-2View Details
691-37-2 -
 4-Methyl-1-pentene CAS 691-37-2View Details
4-Methyl-1-pentene CAS 691-37-2View Details
691-37-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8