4-(isopropyl)-2-[3-(trifluoromethyl)phenyl]morpholine hydrochloride
- CAS NO.:26629-86-7
- Empirical Formula: C14H19ClF3NO
- Molecular Weight: 309.7549696
- MDL number: MFCD01713669
- EINECS: 247-854-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
![4-(isopropyl)-2-[3-(trifluoromethyl)phenyl]morpholine hydrochloride Structural](https://img.chemicalbook.in/CAS/GIF/26629-86-7.gif)
What is 4-(isopropyl)-2-[3-(trifluoromethyl)phenyl]morpholine hydrochloride?
Originator
Conflictan,Sarbach,France,1982
Manufacturing Process
(1) 1,2-Dibromo-2-(2-chloro)ethoxyethane: 640 g of bromine (4 mols) are added dropwise, with stirring, to 426 g (4 mols) of 2-chloroethylvinyl ether dissolved in 1,040 ml of chloroform maintained at -10°C.
When addition is ended, the solvent and then the residue are distilled in vacuum to obtain 690 g of product. Yield = 65%.
(2) 2-(3-Trifluoromethyl)-2-(2-chloro)ethoxy-1-bromoethane: (3Trifluoromethyl)phenyl magnesium bromide is prepared under the normal conditions for magnesium derivatives, from 48.6 g of magnesium turnings and 455.7 g of (3-trifluoromethyl)bromobenzeneand 1.5 liters anhydrous ether.
To the solution of the magnesium compound so obtained the following solution is added dropwise, with stirring so as to maintain a slight reflux of ether: 1,2dibromo-2-(2-chloro)-ethoxyethane: 550 g. Anhydrous ether: 300 ml.
After the addition, reflux heating is continued for two hours, cooling is carried out and there is hydrolysis by the mixture: Ice: 500 g. Concentrated HCl: 200 ml.
The organic phase is decanted, washed in NaCl saturated water and dried on anhydrous Na2SO4; the ether is distilled and the residue is rectified in vacuum to obtain 361 g of the product. Yield = 54%.
According to gas phase chromatography, the product so obtained is about 95% pure and it can be used in further reactions without a second rectification.
(3) 2-(3-Trifluoromethyl)phenyl-4-isopropyl tetrahydro-1,4-oxazine hydrochloride: The following mixture is heated in an autoclave at 100°C; 2-(3trifluoromethyl)-2-(2-chloro)-ethoxy-1-bromoethane: 33.15 g (0.1 mol); isopropylamine: 20 g (0.34 mol); toluene: 100 ml.
After filtration of the isopropylamine hydrochloride and bromohydrate, the solvent is stripped and the residue is admixed with ~ 4 N HCl and the aqueous phase is washed with ether. The aqueous phase is treated with 50% aqueous NaOH, the amine is ether-extracted and, after drying on anhydrous Na2SO4, the ether is distilled and the residue is rectified in vacuum to obtain 14 g of the product. Yield = 50%.
The hydrochloride is crystallized by adding ethyl acetate to the base and then adding the necessary amount of pure alcohol saturated in dry HCl. Melting point 164°C.
Therapeutic Function
Antidepressant
Properties of 4-(isopropyl)-2-[3-(trifluoromethyl)phenyl]morpholine hydrochloride
| Melting point: | 164° |
Safety information for 4-(isopropyl)-2-[3-(trifluoromethyl)phenyl]morpholine hydrochloride
Computed Descriptors for 4-(isopropyl)-2-[3-(trifluoromethyl)phenyl]morpholine hydrochloride
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 3-Nitrobenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
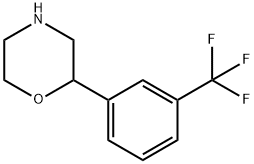
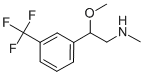
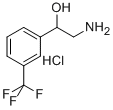
![2-Amino-1-[3-(trifluoromethyl)phenyl]ethanol](https://img.chemicalbook.in/CAS/GIF/21172-28-1.gif)
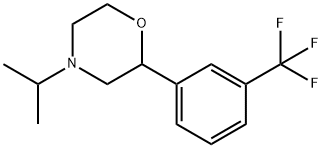
![4-(isopropyl)-2-[3-(trifluoromethyl)phenyl]morpholine hydrochloride](https://img.chemicalbook.in/CAS/GIF/26629-86-7.gif)
You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1