4-Iodophenol
- CAS NO.:540-38-5
- Empirical Formula: C6H5IO
- Molecular Weight: 220.01
- MDL number: MFCD00002327
- EINECS: 208-745-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
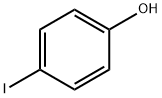
What is 4-Iodophenol?
Chemical properties
Light brown powder. It dissolves slightly in water, but readily dissolves in ethanol, ether, and other organic solvents.
The Uses of 4-Iodophenol
4-Iodophenol is used in chemiluminescence imaging assays and experiments, as diagnostic agents in order to detect cancer cells in patients. Also it is used in the synthesis of agonists towards the est rogen β receptor.
What are the applications of Application
4-Iodophenol is 4-Iodophenol is a chemical used in chemiluminescence imaging assays and experiments, as diagnostic agents in order to detect cancer cells in patients.
Definition
ChEBI: 4-iodophenol is an iodophenol.
Preparation
It is obtained from 4-Aminophenol by diazotisation and substitution.
What are the applications of Application
4-Iodophenol can be used as a building block for the synthesis of:
Hydroxybiaryls by reacting with aryl boronic acids via Pd-catalyzed Suzuki-Miyaura coupling reaction.[1][2]
Aryl substituted olefins by reacting with acrylates via Pd-catalyzed Heck reaction.[3]
Iodinated-4-aryloxymethylcoumarins [4]
Hydroxylated stilbenoids [5] and psammaplysenes A and B.[6]
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 2, p. 355, 1943
The Journal of Organic Chemistry, 70, p. 8590, 2005 DOI: 10.1021/jo051191x
Tetrahedron Letters, 40, p. 6051, 1999 DOI: 10.1016/S0040-4039(99)01236-8
Metabolism
4-Iodophenol has known human metabolites that include (2S,3S,4S,5R)-3,4,5-trihydroxy-6-(4-iodophenoxy)oxane-2-carboxylic acid.
Purification Methods
Crystallise 4-iodophenol from pet ether (b 80-100o) or distil it in vacuo. If the material has a brown or violet color, then dissolve it in CHCl3, shake it with 5% sodium thiosulfate solution until is colourless. Dry (Na2SO4), extract, evaporate and disil the residue in vacuo. [Dains & Eberly Org Synth Coll Vol II 355 1948, Beilstein 6 IV 1074.]
References
[1] HIDEHIRO SAKURAI Toshikazu H Tatsuya Tsukuda. Pd/C as a Reusable Catalyst for the Coupling Reaction of Halophenols and Arylboronic Acids in Aqueous Media[J]. The Journal of Organic Chemistry, 2002, 67 8: 2721-2722. DOI:10.1021/jo016342k.
[2] PIOTR WAWRZYNIAK Joachim H. Microwave-Promoted Suzuki—Miyaura Coupling of Arylboronic Acids with 1-Bromo-2-naphthol, o-Bromophenol, and o-Chlorophenol.[J]. ChemInform, 2007, 38 14. DOI:10.1002/chin.200714105.
[3] LUNXIANG YIN Juergen L. Carbon—Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts[J]. ChemInform, 2007, 38 22. DOI:10.1002/chin.200722237.
[4] MAHANTESHA BASANAGOUDA . Synthesis, structure–activity relationship of iodinated-4-aryloxymethyl-coumarins as potential anti-cancer and anti-mycobacterial agents[J]. European Journal of Medicinal Chemistry, 2014, 74: Pages 225-233. DOI:10.1016/j.ejmech.2013.12.061.
[5] AMIT SHARD. Tandem Heck/Decarboxylation/Heck Strategy: Protecting-Group-Free Synthesis of Symmetric and Unsymmetric Hydroxylated Stilbenoids?[J]. Angewandte Chemie International Edition, 2012, 51 49: 12250-12253. DOI:10.1002/anie.201206346.
[6] SAVVAS N. GEORGIADES Jon C. Total Synthesis of Psammaplysenes A and B, Naturally Occurring Inhibitors of FOXO1a Nuclear Export[J]. Organic Letters, 2005, 7 19: 4091-4094. DOI:10.1021/ol0513286.
Properties of 4-Iodophenol
| Melting point: | 92-94 °C(lit.) |
| Boiling point: | 138 °C5 mm Hg(lit.) |
| Density | 1.8573 |
| Flash point: | 138°C/5mm |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Crystals or Crystalline Powder |
| pka | 9.33(at 25℃) |
| color | Pink or beige to brown |
| Water Solubility | slightly soluble |
| Sensitive | Light Sensitive |
| Merck | 14,5036 |
| BRN | 1904544 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 540-38-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 4-iodo-(540-38-5) |
| EPA Substance Registry System | p-Iodophenol (540-38-5) |
Safety information for 4-Iodophenol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Iodophenol
4-Iodophenol manufacturer
JSK Chemicals
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 4-Iodophenol 98%View Details
4-Iodophenol 98%View Details -
 4-Iodophenol 98%View Details
4-Iodophenol 98%View Details
540-38-5 -
![4-Iodophenol [for Biochemical Research] CAS 540-38-5](https://img.chemicalbook.in//Content/image/CP5.jpg) 4-Iodophenol [for Biochemical Research] CAS 540-38-5View Details
4-Iodophenol [for Biochemical Research] CAS 540-38-5View Details
540-38-5 -
 4-Iodophenol, 98%+ CAS 540-38-5View Details
4-Iodophenol, 98%+ CAS 540-38-5View Details
540-38-5 -
 4-Iodophenol CAS 540-38-5View Details
4-Iodophenol CAS 540-38-5View Details
540-38-5 -
 4-IodophenolView Details
4-IodophenolView Details
540-38-5 -
 4-Iodophenol Cas No.540-38-5View Details
4-Iodophenol Cas No.540-38-5View Details
540-38-5 -
 4-IodophenolView Details
4-IodophenolView Details
540-38-5
