4-Hexylresorcinol
Synonym(s):1,3-Dihydroxy-4-hexylbenzene, 1,3-Dihydroxy-4-hexylbenzene;4-Hexyl-1,3-dihydroxybenzene;4-Hexylresorcinol;Antascarin;Ascarinol
- CAS NO.:136-77-6
- Empirical Formula: C12H18O2
- Molecular Weight: 194.27
- MDL number: MFCD00002284
- EINECS: 205-257-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:34
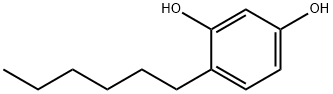
What is 4-Hexylresorcinol?
Absorption
Owing to the poor absorption of hexylresorcinol, systemic exposure and symptoms are unusual .
Toxicity
In the over-the-counter antiseptic and oral analgesic sprays and lozenges that hexylresorcinol is typically employed as the active ingredient, it is noted that overdosage of the agent may cause minor gastrointestinal irritation .
The probable oral LD50 of hexylresorcinol in humans has been estimated to be between 500 and 5000 mg/kg body weight, between 1 oz and 1 pint (or 1 lbs) for a 70 kg person .
Chemical properties
slightly red powder
The Uses of 4-Hexylresorcinol
anthelmintic, topical antiseptic
The Uses of 4-Hexylresorcinol
4-Hexylresorcinol is an inhibitor of transglutaminase-2 in KB cells. Studies indicate that 4-Hexylresorcinol reduces DNA oxidative damage in human lymphocytes. is a substituted dihydroxybenzene used topically as an antiseptic for the treatment of minor skin infections. is a substituted phenol with bactericidal, antihelminthic and potential antineoplastic activities. The combination of 4-Hexylresorcinol and Cisplatin has been shown to inhibit tumor growth, cell growth, and KB cell viability.
The Uses of 4-Hexylresorcinol
4-Hexylresorcinol is used as the starting material to synthesize a potent immune suppressor, celastramycin A. It is a precursor to prepare resorcinol-sn-glycerol derivatives, that exhibit high affinity for cannabinoid type 1 receptor. It can also be incorporated as a linker while building catenanes. p-Hexylresorcinol shows antiimnflammatory activity acting on the human 5-LO (5-Lipoxygenase) proinflammatory enzyme.
Background
Hexylresorcinol is a substituted dihydroxybenzene. It exhibits antiseptic, anthelmintic, and local anesthetic properties. It can be found in topical applications for minor skin infections and in oral solutions or throat lozenges for pain relief and first aid antiseptic.
The compound may also be used commonly in various commercial cosmetic anti-aging creams while ongoing studies research the possibility of using hexylresorcinol as an anti-cancer therapy - indications all of which require further study and testing at the current moment.
What are the applications of Application
4-Hexylresorcinol is a potent irreversible inhibitor of polyphenol oxidase
Indications
Hexylresorcinol is predominantly employed as the active ingredient in lotions, sprays, or lozenges indicated as a (a) topical antiseptic to help prevent skin infection in minor cuts, scrapes, or burns, or (b) as an antiseptic and local anesthetic for the relief of a sore throat and its associated pain .
In addition, hexylresorcinol is used as an active ingredient in various commercial cosmetic skincare products as an anti-aging cream while other studies have looked into whether or not the compound could be used effectively as an anti-inflammatory agent or even as an anti-cancer therapy .
Definition
ChEBI: 4-hexylbenzene-1,3-diol is a member of resorcinols.
General Description
P-hexylresorcinol appears as pale-yellow viscous liquid (that becomes solid on standing at room temperature) or a peach colored powder. Pungent faintly fatty odor. Sharp astringent taste. (NTP, 1992)
Air & Water Reactions
Insoluble in water.
Reactivity Profile
4-Hexyl-1,3-benzenediol may be sensitive to prolonged exposure to light. Incompatible with acid chlorides, acid anhydrides and oxidizing agents .
Hazard
Irritant to respiratory tract and skin, concentrated solutions are vesicant.
Fire Hazard
Flash point data for 4-Hexyl-1,3-benzenediol are not available. 4-Hexyl-1,3-benzenediol is probably combustible.
Biological Activity
hexylresorcinol is a mushroom tyrosinase inhibitor.tyrosinase, a copper-containing enzyme, is distributed in microorganisms, animals, and plants widely. mushroom tyrosinase has been becoming popular due to its availability and usages in various applications.
Pharmacokinetics
Hexylresorcinol is a phenol derivative, and in typical therapeutic usage is primarily a local anesthetic for topical use on the mucous membranes of the mouth and throat . The local anesthetic like properties of hexylresorcinol is likely due to its sodium channel blocking effects . The agent also demonstrates mild antiseptic activity as well as an apparent anti-inflammatory, demulcent action .
Clinical Use
Hexylresorcinol, also known as 4-hexylresorcinol, is a white crystalline substance with a faint phenolic odor. When applied to the tongue it produces a sensation of numbness. It is freely soluble in alcohol but only slightly soluble in water(1–20,000 parts). Hexylresorcinol is an effective antiseptic,possessing both bactericidal and fungicidal properties.The phenol coefficient of hexylresorcinol against S. aureusis 98. As is typical for alkylated phenols, hexylresorcinol possesses surfactant properties. The compound also haslocal anesthetic activity. Hexylresorcinol is formulated into throat lozenges because of its local anesthetic and antisepticproperties. These preparations are probably of little value. Hexylresorcinol (in the concentration in thelozenge) is probably not antiseptic, and the local anestheticproperty can anesthetize the larynx, causing temporary laryngitis.
in vitro
previous results showed hexylresorcinol could inhibit both mono- and di-phenolase activity of mushroom tyrosinase. moreover, hexylresorcinol at 2 μm lengthened the lag period from 98 s to 26. hexylresorcinol could also display reversible inhibition of the enzyme. in addition, the kinetic analyses showed that hexylresorcinol was a competitive inhibitor with the apparent inhibition constant binding with free enzyme to be 0.443 μm for diphenolase [1].
in vivo
previous in vivo study showed that hexylresorcinol could induce chromosome aberrations in mouse eukaryotic cells at doses of 0.5, 0.05, and 0.005 mg/g and the metabolic transformation of hexylresorcinol decreased its genotoxic effect in mice. moreover, the mutagenic effect lasted for 3 days only at the highest dose of hexylresorcinol (0.5 mg/g). thus, hexylresorcinol doses less than 0.5 mg/g were metabolized within two days to the extent of the cytotoxic effect. in addition, hexylresorcinol was transformed at a rate of 0.0025–0.025 mg/day after a single administration to mice [2].
Metabolism
Regarding the metabolism of hexylresorcinol, it has been reported that excretion of the compound in the urine is largely in the form of an ethereal sulfate conjugate .
References
[1] chen qx,ke ln,song kk,huang h,liu xd. inhibitory effects of hexylresorcinol and dodecylresorcinol on mushroom (agaricus bisporus) tyrosinase. protein j.2004 feb;23(2):135-41.
[2] margulis ab,ozhiganova iv,bushmanova ov,kolpakov ai,il'inskaia on. hexylresorcinol induces chromosome aberrations in mouse peripheral blood cells. genetika. 2005 aug;41(8):1045-8.
Properties of 4-Hexylresorcinol
| Melting point: | 65-67 °C (lit.) |
| Boiling point: | 333-335 °C (lit.) |
| Density | 1.0010 (rough estimate) |
| vapor pressure | 0.001Pa at 25℃ |
| refractive index | 1.5050 (estimate) |
| Flash point: | >100°C |
| storage temp. | 2-8°C |
| solubility | chloroform: 50 mg/mL, clear |
| form | Powder and/or Chunks |
| pka | pKa 9.1 (Uncertain) |
| color | White to pale orange or pale pink |
| PH | 7.7 (0.5g/l, H2O, 20℃) |
| Water Solubility | 0.05 g/100 mL (18 ºC) |
| Merck | 14,4712 |
| BRN | 2048312 |
| Stability: | Stable. Incompatible with acid chlorides, acid anhydrides, oxidizing agents. |
| CAS DataBase Reference | 136-77-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Hexylresorcinol(136-77-6) |
| EPA Substance Registry System | 4-Hexylresorcinol (136-77-6) |
Safety information for 4-Hexylresorcinol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Hexylresorcinol
| InChIKey | WFJIVOKAWHGMBH-UHFFFAOYSA-N |
4-Hexylresorcinol manufacturer
SNA Healthcare Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 4-Hexylresorcinol 98%View Details
4-Hexylresorcinol 98%View Details -
 4-Hexylresorcinol CAS 136-77-6View Details
4-Hexylresorcinol CAS 136-77-6View Details
136-77-6 -
 4-Hexyl resorcinol CAS 136-77-6View Details
4-Hexyl resorcinol CAS 136-77-6View Details
136-77-6 -
 Hexylresorcinol CAS 136-77-6View Details
Hexylresorcinol CAS 136-77-6View Details
136-77-6 -
 4 N Hexyl Resorcinol powder, Packaging Size: 25 kgView Details
4 N Hexyl Resorcinol powder, Packaging Size: 25 kgView Details
136-77-6 -
 4-Hexylresorcinol chemical CAS No: 136-77-6View Details
4-Hexylresorcinol chemical CAS No: 136-77-6View Details
136-77-6 -
 4 HexylResorcinol chemicalView Details
4 HexylResorcinol chemicalView Details
136-77-6 -
 4-Hexylresorcinol CAS 136 77 6, Packaging Size: 25 kgView Details
4-Hexylresorcinol CAS 136 77 6, Packaging Size: 25 kgView Details
136-77-6
