4-Butylresorcinol
- CAS NO.:18979-61-8
- Empirical Formula: C10H14O2
- Molecular Weight: 166.22
- MDL number: MFCD01684800
- EINECS: 606-191-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
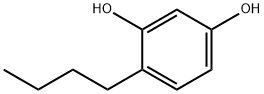
What is 4-Butylresorcinol?
Description
4-Butylresorcinol is one of the best ingredients for treating hyperpigmentation and facilitating skin-lightening in the cosmetic industry. It even works wonders for melasma treatment which is the condition of dark patches appearing on the face due to hormonal changes during pregnancy.
The Uses of 4-Butylresorcinol
4-Butylresorcinol is recognized as a potential inhibitor of Cytochrome P450, an enzyme complex that plays a crucial role in the metabolism of various substances in the body. Its ability to inhibit tyrosinase, a key enzyme in melanin production, results in hypopigmentation, which is a lightening or loss of skin color.
What are the applications of Application
Glycerol is used both in sample preparation and gel formation for polyacrylamide gel electrophoresis. Glycerol (5-10%) increases the density of a sample so that the sample will layer at the bottom of a gel′s sample well. Glycerol is also used to aid in casting gradient gels and as a protein stabilizer and storage buffer component.
Definition
ChEBI: 4-n-Butylresorcinol is a member of resorcinols.
Biological Activity
Glutathione reductase IGR) is a crucial flavoenzyme in the antioxidant defense system. Reduced glutathione (GSH) is used by glutathione peroxidase to detoxify hydrogen peroxide and in the process is converted to oxidized glutathione (GSSG). The GSSG is then recycled back to GSH by glutathione reductase (GR) using NADPH that is then converted to NADP+. The regenerated GSH is then available to detoxify more hydrogen peroxide. The enzyme uses FAD as a cofactor. GR and glutathione peroxidase may inhibit lipid peroxidation by functioning as antioxidant enzymes in sperm. Glutathione reductase shares a structural motif with a number of other proteins including aspartyl proteases, citrate synthase, EF hands, hemoglobins, lipocalins, and α/β hydrolases. GR is stimulated by melatonin and is reportedly irreversibly inhibited by a number of oxygen radical generating systems.A sweet tastant for mammals. A glycerol taste receptor binding site specific for glucose has been proposed in drosophila.
Mechanism of action
The mechanism of action of 4-butylresorcinol depigmentation is through inhibition of tyrosinase and tyrosinase-related protein-1 (TRP-1)[1].
Synthesis

Under the protection of nitrogen, 110 g (1 mol) of resorcinol and 220 g of n-heptane were mixed in the reaction flask, heated and dissolved, and the temperature was lowered to 10 ?? C. A turbid solution of 40.8 g (1.02 mol) of sodium hydroxide and 100 g of n-butanol was added in portions. , And control the temperature of 10 to 15 , after TLC (EA developing agent) detection of almost no remaining raw materials, add n-butanol (2.5mol), tris (pentafluorobenzene) borane 17.5g (0.1mol) and calcium chloride 1.1 g, raise the temperature to 40-45 ?? C for 1 hour, then raise the temperature to 98 ?? C for reflux and water separation. After 8 hours of water separation, take a sample and quench the GC to detect resorcinol <1%, cool to room temperature, add a small amount of water to quench It is extinguished, and then dilute sulfuric acid is added to adjust the pH = 1-2. The insoluble solids are filtered through diatomaceous earth, and the organic phase is concentrated to a stagnant liquid. Water is added for replacement, and then 660 g of water, 2.2 g of sodium thiosulfate, and 5.5 g are added. Activated carbon was heated to reflux for 1 hour, and was hot-filtered to obtain a pale yellow solution. Then, flaky crystals were precipitated by cooling, and 151 g of 4-n-butylresorcinol was obtained by filtration, GC: 99.3%, and yield: 90.9%.
References
[1] D. RESENDE. Skin Depigmenting Agents in Anti-Aging Cosmetics: A Medicinal Perspective on Emerging Ingredients[J]. Applied Sciences-Basel, 2022. DOI:10.3390/app12020775.
Properties of 4-Butylresorcinol
| Melting point: | 50.0 to 55.0 °C |
| Boiling point: | 166°C/7mmHg(lit.) |
| Density | 1.092±0.06 g/cm3(Predicted) |
| vapor pressure | 0.041Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO : 150 mg/mL (902.42 mM) |
| pka | 9.95±0.18(Predicted) |
| form | Powder |
| color | white to pale yellow powder |
| Odor | Characteristic |
| InChI | InChI=1S/C10H14O2/c1-2-3-4-8-5-6-9(11)7-10(8)12/h5-7,11-12H,2-4H2,1H3 |
| CAS DataBase Reference | 18979-61-8(CAS DataBase Reference) |
Safety information for 4-Butylresorcinol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P501:Dispose of contents/container to..… |
Computed Descriptors for 4-Butylresorcinol
| InChIKey | CSHZYWUPJWVTMQ-UHFFFAOYSA-N |
| SMILES | C1(O)=CC=C(CCCC)C(O)=C1 |
4-Butylresorcinol manufacturer
SPARKVEE FINE CHEMICALS PRIVATE LIMITED
VJ CHEMTRADE PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 4-Butylresorcinol CAS 18979-61-8View Details
4-Butylresorcinol CAS 18979-61-8View Details
18979-61-8 -
 18879-61-8 4-N-BUTYLRESORCINOL 99%View Details
18879-61-8 4-N-BUTYLRESORCINOL 99%View Details
18879-61-8 -
 Powder 4 N Butyl Resorcinol, Packaging Size: 5 kgView Details
Powder 4 N Butyl Resorcinol, Packaging Size: 5 kgView Details
18979-61-8 -
 99% Powder 4 N Butyl Resorcinol, 18979-61-8View Details
99% Powder 4 N Butyl Resorcinol, 18979-61-8View Details
18979-61-8 -
 4 Butyl Resorcinol, Packaging Size: 25 kgView Details
4 Butyl Resorcinol, Packaging Size: 25 kgView Details
18979-61-8 -
 4 Butyl Resorcinol, Packaging Size: 1 kgView Details
4 Butyl Resorcinol, Packaging Size: 1 kgView Details
18979-61-8 -
 4-N-BUTYL RESORCINOL (18979-61-8), Packaging Size: 200 kgView Details
4-N-BUTYL RESORCINOL (18979-61-8), Packaging Size: 200 kgView Details
18979-61-8 -
 4 N Butyl Resorcinol powder, Packaging Size: 50 KgsView Details
4 N Butyl Resorcinol powder, Packaging Size: 50 KgsView Details
18979-61-8
