3-Hydroxy-2-methyl-4H-pyran-4-one
Synonym(s):3-Hydroxy-2-methyl-4H-pyran-4-one;3-Hydroxy-2-methyl-4-pyrone;Larixinic acid;Maltol
- CAS NO.:118-71-8
- Empirical Formula: C6H6O3
- Molecular Weight: 126.11
- MDL number: MFCD00006578
- EINECS: 204-271-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-21 19:13:37
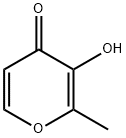
What is 3-Hydroxy-2-methyl-4H-pyran-4-one?
Description
Maltol has a warm, sweet, fruity odor and a jam-like odor in solution. It may be prepared by alkaline hydrolysis of streptomycin salts; also from piperidine to pyromeconic acid and subsequent methylation at the 2 position.
Description
Stout beer and some coffees are good sources of maltol, an antioxidant found in chicory and roasted malt. Widely used as a flavoring agent, maltol confers a fragrant, caramel-like odor to many foods such as breads and cakes.
Chemical properties
White, crystalline powder; characteristic caramel-butterscotch odor and suggestive of a fruity-strawberry aroma in dilute solution. Melting range 160–164C. Slightly soluble in water; more soluble in alcohol and propylene glycol.
Chemical properties
White crystalline solid with a characteristic, caramel-like odor and taste. In dilute solution it possesses a sweet, strawberry-like or pineapple-like flavor and odor.
Chemical properties
Maltol occurs in pine needles and the bark of young larch trees. It is produced when cellulose or starch is heated and is a constituent of wood tar oils. It forms crystals (mp 162–164°C) with a caramel-like odor, reminiscent of freshly baked cakes.
Chemical properties
Maltol has a caramel–butterscotch odor and in solution it has a jam-like odor. This compound is also reported to have a suggestive of fruity, strawberry aroma in dilute solution.
Occurrence
Reported found in the bark of young larch trees (Pinus larix), pine needles (Abies alba), chicory, wood tars and oils, and roasted malt. Also reported found in wheat and rye bread, milk, butter, smoked pork, beer, cocoa, coffee, roasted barley, filberts, peanuts, soybean, beans, tamarind, licorice, sake, malt, dried bonito, clam and cocoa liquor.
The Uses of 3-Hydroxy-2-methyl-4H-pyran-4-one
A fragrance molecule used in flavor enhancers and fragrances.
The Uses of 3-Hydroxy-2-methyl-4H-pyran-4-one
Flavoring agent, to impart "freshly baked" odor and flavor to bread and cakes.
The Uses of 3-Hydroxy-2-methyl-4H-pyran-4-one
Maltol may be used as an analytical reference standard for the quantification of the analyte in:
- Synthetic and commercial food samples using UV–Vis spectrophotometry with chemometrics methods.
- Food and beverage matrices using FIA-direct chemiluminescence procedure.
Definition
ChEBI: A natural product found in Cordyceps sinensis.
Production Methods
Maltol is mainly isolated from naturally occurring sources such as beechwood and other wood tars; pine needles; chicory; and the bark of young larch trees. It may also be synthesized by the alkaline hydrolysis of streptomycin salts or by a number of other synthetic methods.
Preparation
Maltol may be produced synthetically starting from kojic acid . Alternatively, it can be isolated from beechwood tar or from extracts of needles from the genus Abies. Commercially available extracts fromAbies balsamea needles, which are also used as flavor and fragrance materials, usually contain 3–8% maltol. It is used in aroma compositions with a caramel note and as a taste intensifier in, for example, fruit flavors (particularly in strawberry flavor compositions).
Aroma threshold values
Detection: 29 ppb
Taste threshold values
Taste characteristics at 100 ppm: sweet, caramellic, cotton candy, with jamy fruity and berry notes.
Synthesis Reference(s)
The Journal of Organic Chemistry, 45, p. 1109, 1980 DOI: 10.1021/jo01294a037
Tetrahedron Letters, 17, p. 1363, 1976 DOI: 10.1016/S0040-4039(00)78065-8
Toxicity evaluation
The acute oral LD50 in rats was reported to be 2.33 g/kg (1.57-3.09 g/kg) (Moreno, 1974). The acute oral 7-day LD50s in mice, rats and chicks were reported to be 848, 1440 and 3720 mg/kg, respectively (Gralla, Stebbins, Coleman & Delahunt, 1969). Acute oral LD50 values were found to be 550 mg/kg in mice, 1620 mg/kg in rabbits and 1410 mg/kg in guinea-pigs (Dow Chemical Company, 1967). The acute sc LD50 in mice was found to be 820 mg/kg. Sc injection of 400 mg/kg resulted in decreased spontaneous activity, bradycardia, hypothermia, skeletal-muscle relaxation and diminution of pinna, corneal, and ipsilateral flexor reflexes (Aoyagi, Kimura & Murata, 1974). Because of a lack of sample, 5 g/kg could only be applied to one rabbit in the dermal LD50 study, but this dosage was not lethal in the one rabbit (Moreno, 1974).
General Description
White crystalline powder with a fragrant caramel-butterscotch odor. pH (5% aqueous solution) 5.3.
Air & Water Reactions
May be sensitive to prolonged exposure to light and air. Somewhat soluble in water at room temperature. Freely soluble in hot water [Merck]. Slightly soluble in cold water.
Reactivity Profile
3-Hydroxy-2-methyl-4H-pyran-4-one is weakly acidic. Reacts with bases. May react with reducing agents. Volatile with steam.
Fire Hazard
Flash point data on 3-Hydroxy-2-methyl-4H-pyran-4-one are not available; however, 3-Hydroxy-2-methyl-4H-pyran-4-one is probably combustible.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Maltol is used in pharmaceutical formulations and food products as a flavoring agent or flavor enhancer. In foods, it is used at concentrations up to 30 ppm, particularly with fruit flavorings, although it is also used to impart a freshly baked odor and flavor to bread and cakes. When used at concentrations of 5–75 ppm, maltol potentiates the sweetness of a food product, permitting a reduction in sugar content of up to 15% while maintaining the same level of sweetness. Maltol is also used at low levels in perfumery.
Pharmacology
In mice, spontaneous motor activity was depressed by sc injection oi relatively low doses of maltol (75 mg/kg), hexobarbitone sleeping time was prolonged by sc or oral administration of 300 mg/kg, and convulsions induced by pentylenetetrazole or strychnine were inhibited by sc injection of toxic doses (500 mg/kg), but 1 mM concentrations of maltol had no effect on oxygen uptake by slices of the brain cortex of the rat (Aoyagi et al. 1974).
Safety Profile
Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. A skin irritant. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Maltol is generally regarded as an essentially nontoxic and
nonirritant material. In animal feeding studies, it has been shown
to be well tolerated with no adverse toxic, reproductive, or
embryogenic effects observed in rats and dogs fed daily intakes of
up to 200mg/kg body-weight of maltol, for 2 years.The WHO
has set an acceptable daily intake for maltol at up to 1mg/kg body-weight.A case of allergic contact dermatitis, attributed to the
use of maltol in a lip ointment, has been reported.
LD50 (chicken, oral): 3.72 g/kg
LD50 (guinea pig, oral): 1.41 g/kg
LD50 (mouse, oral): 0.85 g/kg
LD50 (mouse, SC): 0.82 g/kg
LD50 (rabbit, oral): 1.62 g/kg
LD50 (rat, oral): 1.41 g/kg
Synthesis
By alkaline hydrolysis of streptomycin salts; also from piperdine to pyromeconic acid and subsequent methylation at the 2 position.
Metabolism
Maltol is rapidly and extensively metabolized in the dog and excreted by the conjugation pathway common to phenolic compounds. Rennhard (1971) reported that 57% of an iv dose was recovered in 24 hr, 88% of the total excretion occurring in the first 6 hr and 65-70% of the dose administered being recovered as sulphate and glucuronide conjugates
storage
Maltol solutions may be stored in glass or plastic containers. The bulk material should be stored in a well-closed container, protected from light, in a cool, dry place.
Purification Methods
It crystallises from CHCl3, toluene, aqueous 50% EtOH or H2O, and is volatile in steam. It can be readily sublimed in a vacuum. It forms a Cu2+ complex. [Beilstein 17 III/IV 5916, 18/1 V 114.]
Incompatibilities
Concentrated solutions in metal containers, including some grades of stainless steel, may discolor on storage.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (oral solutions and syrups). Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of 3-Hydroxy-2-methyl-4H-pyran-4-one
| Melting point: | 160-164 °C(lit.) |
| Boiling point: | 205 °C |
| Density | 1.046 g/mL at 25 °C |
| vapor pressure | 0.15Pa at 24℃ |
| FEMA | 2656 | MALTOL |
| refractive index | n |
| Flash point: | 198 °F |
| storage temp. | 2-8°C |
| solubility | methanol: 50 mg/mL, clear |
| form | Liquid |
| pka | 8.41±0.10(Predicted) |
| color | Clear colorless |
| PH | 5.3 (0.5g/l, H2O) |
| Odor | at 5.00 % in benzyl alcohol. sweet caramel cotton candy jam fruity baked bread |
| explosive limit | 25% |
| Water Solubility | 1.2 g/100 mL (25 ºC) |
| Merck | 14,5713 |
| JECFA Number | 1480 |
| BRN | 112169 |
| CAS DataBase Reference | 118-71-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-Hydroxy-2-methyl-4h-pyran-4-one(118-71-8) |
| EPA Substance Registry System | Maltol (118-71-8) |
Safety information for 3-Hydroxy-2-methyl-4H-pyran-4-one
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for 3-Hydroxy-2-methyl-4H-pyran-4-one
| InChIKey | XPCTZQVDEJYUGT-UHFFFAOYSA-N |
3-Hydroxy-2-methyl-4H-pyran-4-one manufacturer
New Products
2,3-Dihydro-5,6-dimethoxy-2-(4-piperidinylmethyl)-1H-inden-1-one hydrochloride 3-Pyridineacrylic acid 2-pyridineacetonitrile Methyl 4-chloropyridine-2-carboxylate hydrochloride 1-Methyl-4-piperidinone 1-Boc-4-cyanopiperidine 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt Ethyl Methanesulfonate N Ethylmethylamine N N N'Trimethyl ethylenediamine (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Boc-his(trt)-OH Sacubitril- Valsartan Ramipril Fmoc-L-Glu-OtBu Boc LeucineRelated products of tetrahydrofuran


![3-[(2,6-DICHLOROBENZYL)OXY]-2-METHYL-4H-PYRAN-4-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB6345738.gif)

![3-[(2,4-DICHLOROBENZYL)OXY]-2-METHYL-4H-PYRAN-4-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB4443476.gif)
You may like
-
 3-Hydroxy-2-methyl-4-pyrone CAS 118-71-8View Details
3-Hydroxy-2-methyl-4-pyrone CAS 118-71-8View Details
118-71-8 -
 Maltol, 99% CAS 118-71-8View Details
Maltol, 99% CAS 118-71-8View Details
118-71-8 -
 Maltol 98% CAS 118-71-8View Details
Maltol 98% CAS 118-71-8View Details
118-71-8 -
 Maltol 99% CAS 118-71-8View Details
Maltol 99% CAS 118-71-8View Details
118-71-8 -
 Maltol CAS 118-71-8View Details
Maltol CAS 118-71-8View Details
118-71-8 -
 Maltol CAS 118-71-8View Details
Maltol CAS 118-71-8View Details
118-71-8 -
 Maltol CAS 118-71-8View Details
Maltol CAS 118-71-8View Details
118-71-8 -
 3-Hydroxy-2-methyl-4-pyrone CAS 118-71-8View Details
3-Hydroxy-2-methyl-4-pyrone CAS 118-71-8View Details
118-71-8
