3-ETHYLAMINOPHENOL
- CAS NO.:621-31-8
- Empirical Formula: C8H11NO
- Molecular Weight: 137.18
- MDL number: MFCD00059617
- EINECS: 210-678-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
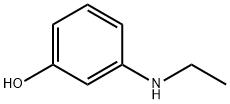
What is 3-ETHYLAMINOPHENOL?
The Uses of 3-ETHYLAMINOPHENOL
3-(Ethylamino)phenol can be used for hair dye compositions.
General Description
Very viscous deep orange liquid.
Air & Water Reactions
3-ETHYLAMINOPHENOL may be sensitive to prolonged exposure to air. . Slightly soluble in water.
Reactivity Profile
An amine and phenol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Phenols do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid.
Fire Hazard
Flash point data for 3-ETHYLAMINOPHENOL are not available, however, 3-ETHYLAMINOPHENOL is probably combustible.
Properties of 3-ETHYLAMINOPHENOL
| Melting point: | 62°C |
| Boiling point: | 252.03°C (rough estimate) |
| Density | 1.0630 (rough estimate) |
| refractive index | 1.5560 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 10.36±0.10(Predicted) |
| color | Light Brown to Very Dark Red |
| EPA Substance Registry System | 3-(Ethylamino)phenol (621-31-8) |
Safety information for 3-ETHYLAMINOPHENOL
Computed Descriptors for 3-ETHYLAMINOPHENOL
3-ETHYLAMINOPHENOL manufacturer
SUMUKHA LIFESCIENCES
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
![2-[(2-amino-5-methylph2-[(2-amino-5-methylphenoxy)methyl]-6-methoxy-8-aminoquinoline-n,n,n',n'-tetraacetic acid tetrapotassium saltenoxy)methyl]-6-methoxy-8-aminoquinoline-n,n,n](https://img.chemicalbook.in/CAS/GIF/73630-23-6.gif)
You may like
-
 621-31-8 3-(Ethylamino)phenol 98%View Details
621-31-8 3-(Ethylamino)phenol 98%View Details
621-31-8 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
