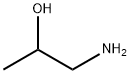
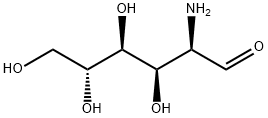
3-Aminopropanol
Synonym(s):3-Amino-1-propanol;3-Aminopropyl alcohol
- CAS NO.:156-87-6
- Empirical Formula: C3H9NO
- Molecular Weight: 75.11
- MDL number: MFCD00008223
- EINECS: 205-864-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30

What is 3-Aminopropanol?
Chemical properties
clear colourless to very slightly yellow liquid
The Uses of 3-Aminopropanol
3-Amino-1-propanol is used in the production of anionic emulsifiers, nonionic polyethylene emulsions. Its salts such as hydrochloride, nitrate, sulfate, phosphate are used for the balanced curing action with selected textile resins. It is used as a starting material in the preparation of beta-lactam antibiotics, humectants for foods and cosmetics. It acts as a corrosion inhibitor and finds application in water treatment, metal treatment and absorption of carbon dioxide gas.
The Uses of 3-Aminopropanol
3-Amino-1-propanol was used in the synthesis of di-tert-butyl aminopropanol derivative.
The Uses of 3-Aminopropanol
Organic intermediate.
Definition
ChEBI: 3-aminopropan-1-ol is a member of the class of propanolamines that is propane with a hydroxy substituent at C-1 and an amino substituent at C-2, making it both a primary amine and a primary alcohol. It is a primary alcohol, a primary amine and a propanolamine.
General Description
Colorless to pale yellow liquid with a fishy odor. Less dense than water. Melting point 12.4°C (54°F). Moderately toxic by ingestion.
Air & Water Reactions
Water soluble.
Reactivity Profile
3-Aminopropanol neutralizes acids to form salts plus water in an exothermic reaction. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Reacts with strong reducing agents, such as hydrides to generate flammable gaseous hydrogen.
Hazard
Irritant to tissue.
Health Hazard
If inhaled may be harmful. Contact may cause burns to skin and eyes. (Organic base.)
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion and skin contact. An experimental teratogen. A severe skin and eye irritant. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx. See also AMINES.
Properties of 3-Aminopropanol
| Melting point: | 10-12 °C (lit.) |
| Boiling point: | 184-187 °C (lit.) |
| Density | 0.982 g/mL at 20 °C (lit.) |
| vapor density | 2.59 (vs air) |
| vapor pressure | 2.1 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 175 °F |
| storage temp. | Store below +30°C. |
| solubility | water: soluble |
| form | Liquid |
| pka | pK1: 9.96(+1) (25°C) |
| Specific Gravity | 0.990 (20/4℃) |
| color | Clear colorless to very slightly yellow |
| PH | 11.6 (10g/l, H2O, 20℃) |
| explosive limit | 2.5-10.6%(V) |
| Water Solubility | miscible |
| Sensitive | Hygroscopic |
| BRN | 741855 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 156-87-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Propanol, 3-amino-(156-87-6) |
| EPA Substance Registry System | 3-Aminopropanol (156-87-6) |
Safety information for 3-Aminopropanol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-Aminopropanol
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 156-87-6 3-Hydroxy propylamine 99%View Details
156-87-6 3-Hydroxy propylamine 99%View Details
156-87-6 -
 3-Amino-1-propanol CAS 156-87-6View Details
3-Amino-1-propanol CAS 156-87-6View Details
156-87-6 -
 3-Amino-1-propanol, 99% CAS 156-87-6View Details
3-Amino-1-propanol, 99% CAS 156-87-6View Details
156-87-6 -
 3-AMINO-1-PROPANOL For Synthesis CAS 156-87-6View Details
3-AMINO-1-PROPANOL For Synthesis CAS 156-87-6View Details
156-87-6 -
 3-Amino-1-propanol CAS 156-87-6View Details
3-Amino-1-propanol CAS 156-87-6View Details
156-87-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1