Menbutone
- CAS NO.:3562-99-0
- Empirical Formula: C15H14O4
- Molecular Weight: 258.27
- MDL number: MFCD00021539
- EINECS: 222-631-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
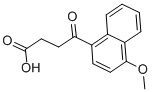
What is Menbutone?
Originator
Hepalande, Delalande ,W. Germany ,1977
The Uses of Menbutone
Menbutone is a cholerectic compound which is used for stimulating gastro-intestinal function by promoting secretory processes in animals including that of cattle, sheep, goats, horses and dogs. It is used in treatment of hepatic dysfunction of dogs by promoting bile secretion.
Definition
ChEBI: Menbutone is a butanone.
Manufacturing Process
395 parts of (α-methoxynaphthalene and 265 parts of succinic anhydride are dissolved in 8,000 parts of dry benzene at room temperature. The resulting solution is stirred and 710 parts of anhydrous aluminum chloride are added over a period of twenty minutes. During the addition the temperature of the reaction mixture rises to about 60°C to 70°C. After the addition the reaction mixture is stirred for fifteen or twenty minutes at 60°C to 70°C and then refluxed for one hour. The hot reaction mixture is then poured onto a mixture of 5,000 parts of ice and 900 parts of concentrated hydrochloric acid. The benzene is removed by steam distillation and the hot aqueous residue is filtered to remove the insoluble β-(1-methoxy-4-naphthoyl)-propionic acid. The residue of the latter is dried and then dissolved in 16,000 parts of hot water containing 300 parts of sodium carbonate. The hot solution is treated with activated charcoal, filtered while hot, chilled and acidified. The residue of purified acid is collected on a filter, washed with water, and dried at 65°C. A yield of 552 parts of purified β-(1-methoxy)-4-naphthoyl)propionic acid, melting at 172°C to 173°C is obtained.
Therapeutic Function
Choleretic
Synthesis
Menbutone is prepared by the reaction of succinic acid anhydride and 1-Methoxynaphthalene. The steps are as follows:
15.8 g of 1-methoxynaphthalene and 10.0 g of succinic anhydride were dissolved in 120 mL of dichloromethane,Stir, Cooling to 1 ~ 3 ° C, Divided into three batches of anhydrous aluminum trichloride 15.0 grams, The addition process takes about 20 minutes, The solution was then heated to 35 ± 2 ° C, Insulation reaction 6 hours (5.5 ~ 6.5 hours range), After the reaction is complete, The reaction solution was poured into an ice-water mixture (200 g of ice and 300 g of water) for 30 minutes,Standing, analysiscrystal, Filter, The filtrate was heated and distilled to recover dichloromethane, The cake is the crude of the ketone ketone; And then the crude ketoprofen water as a solvent by 2-3 times recrystallization, Activated carbon decolorization, Demon ketone boutique. The mass of the present product of the compound was 22.3 g, Melting point of 176 ~ 179 ° C, The yield was 86.4%.
Properties of Menbutone
| Melting point: | 172-173° |
| Boiling point: | 361.52°C (rough estimate) |
| Density | 1.2105 (rough estimate) |
| refractive index | 1.4872 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly, Sonicated), Ethanol (Slightly, Heated) |
| pka | 4.44±0.17(Predicted) |
| form | Solid |
| color | White to Off-White |
| InChI | InChI=1S/C15H14O4/c1-19-14-8-6-11(13(16)7-9-15(17)18)10-4-2-3-5-12(10)14/h2-6,8H,7,9H2,1H3,(H,17,18) |
Safety information for Menbutone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Menbutone
| InChIKey | FHGJSJFIQNQBCK-UHFFFAOYSA-N |
| SMILES | C12C=CC=CC1=C(OC)C=CC=2C(=O)CCC(=O)O |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1