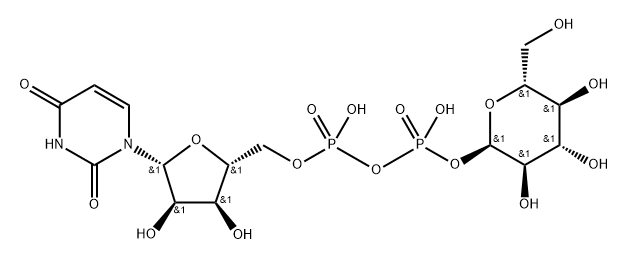
(2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic
- CAS NO.:2616-64-0
- Empirical Formula: C15H22N2O18P2
- Molecular Weight: 580.29
- MDL number: MFCD15145135
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-21 14:41:48
![(2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic Structural](https://img.chemicalbook.in/CAS/20210111/GIF/2616-64-0.gif)
What is (2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic?
Description
An intermediate in the phase II reaction that results in the formation of glucuro_x0002_nic acid conjugates of xenobiotics which contain substituents such as hydroxyl, amino, or sulfhydryl groups, forming the O-, N-, and S-glucuronides, respectively. UDPGA is formed by two reactions: (i) the formation of UDPG from UTP and glucose 1-phosphate and (ii) the formation of UDPGA from UDPG. The two reactions are catalyzed by UDPG pyrophosphorylase and UDPG dehydrogenase, respectively, whereas glucuronide formation is catalyzed by glucuronosyltransferase. Glucuronide formation from xenobiotics is common in all animal groups except insects.
Definition
ChEBI: A UDP-sugar having alpha-D-glucuronic acid as the sugar component.
Properties of (2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic
| Density | 2.05±0.1 g/cm3(Predicted) |
| pka | 1.10±0.50(Predicted) |
| CAS DataBase Reference | 2616-64-0 |
Safety information for (2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic
Computed Descriptors for (2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic
New Products
Tetrabutylammonium hydrogen sulfate 2,2,6-Trimethyl-4H-1,3-dioxin-4-one 4-Piperidinopiperidine tert-Butyl acetoacetate 4-BROMOMETHYLTETRAHYDROPYRAN 3-Hydroxyazetidine hydrochloride Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) 3-Methoxybenzonitrile 4-Methoxybenzonitrile Dibenzoyl Peroxide Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran
You may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.
