D-Glucuronic acid
Synonym(s):D -Glucuronic acid;Glucosiduronic acid
- CAS NO.:6556-12-3
- Empirical Formula: C6H10O7
- Molecular Weight: 194.14
- MDL number: MFCD00077778
- EINECS: 229-486-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
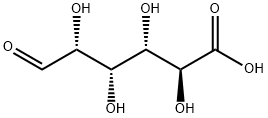
What is D-Glucuronic acid?
Description
Glucuronic acid (from Ancient Greek γλυκ?? "sweet" + ο?ρον "urine") is a carboxylic acid. Its structure is similar to that of glucose. However, glucuronic acid's sixth carbon is oxidized to a carboxylic acid. Its formula is C6H10O7. The salts and esters of glucuronic acid are known as glucuronates; the anion C6H9O7-is the glucuronate ion.
Chemical properties
White Solid
The Uses of D-Glucuronic acid
- D-Glucuronic acid is widely distributed in the plant and animal kingdoms. D-Glucuronic acid usually occurs in "paired" form as a glycosidic combination with phenols, alcohols. Such glucuronides form in the liver to detoxify poisonous hydroxyl-containing substances. The glucuronides present in normal urine are those of phenol, cresol, and indoxyl. After the ingestion of poisons such as morphine, chloral hydrate, camphor, or turpentine, glucuronides formed with the poison or its hydroxylated derivatives appear in the urine.
- Chiral D-Glucuronic acid is a basic building block of hyaluronic acid and chondroitin sulfate. It is a precursor to vitamin C, the chief detoxifying agent in plants and animals.
- D-Glucuronic Acid is a sugar acid formed by the oxidation of the C-6 carbon of GLUCOSE. In addition to being a key intermediate metabolite of the uronic acid pathway, glucuronic acid also plays a role in the detoxification of certain drugs and toxins by conjugating with them to form GLUCURONIDES.
The Uses of D-Glucuronic acid
D-glucuronic acid and D-galacturonic acid are naturally occurring hexuronic acids present in glycosaminoglucans, glucuronide conjugates in mammals and in plant cell wall polysaccharides.
D-Glucuronic acid exists as a component of glycosaminoglucans such as hyaluronan, heparin and chondroitin sulphate present in mammalian connective tissue such as cartilage. In the synthesis of glucuronide conjugates in mammals glucuronic acid is conjugated to xenobiotic compounds, a biosynthesis reaction known as glucuronidation, catalysed by the enzyme UDP-glucuronyltransferase and considered a detoxifying process.
Both D-glucuronic acid and D-galacturonic acid are major components of plant cell wall polysaccharides. D-glucuronic acid is a component of arabinoxylan, which consists of a β-(1,4)-linked xylan backbone substituted with α-(1-2/3)-linked L-arabinofuranose and α-(1-2)- linked 4-O-methylglucuronic acid. D-Galacturonic acid is the major component of pectin comprising the α-(1,4)-linked galacturonan backbone of homogalacturonan and rhamnogalacturonan II, and is present within the repeating disaccharide unit [,4)-α-D-GalpA-(1,2)-α- L-Rhap-(1,] of rhamnogalacturonan I.
The Uses of D-Glucuronic acid
D-Glucuronic acid is used as pharmaceutical intermediate and in chemical research.
Definition
ChEBI: A D-glucopyranuronic acid in which the anomeric centre has alpha-configuration.
Definition
glucuronic acid: A compound,OC6H9O6, derived from the oxidationof glucose. It is an important constituentof gums and mucilages. Glucuronicacid can combine withhydroxyl (–OH), carboxyl (–COOH), oramino (-NH2) groups to form a glucuronide.The addition of a glucuronidegroup to a molecule(glucuronidation) generally increasesthe solubility of a compound; henceglucuronidation plays an importantrole in the excretion of foreign substances.
Biological Functions
Proteoglycans Glucuronic acid is common in carbohydrate chains of proteoglycans. It is part of mucous animal secretions (such as saliva), cell glycocalyx and intercellular matrix (for instance hyaluronan))
Glucuronidation of toxic substances
In the animal body, glucuronic acid is often linked to the xenobiotic metabolism of substances such as drugs, pollutants, bilirubin, androgens, estrogens, mineralocorticoids, glucocorticoids, fatty acid derivatives, retinoids, and bile acids. These linkages involve glycosidic bonds, and this linkage process is known as glucuronidation. Glucuronidation occurs mainly in the liver, although the enzyme responsible for its catalysis, UDP-glucuronyltransferase, has been found in all major body organs, e.g., intestine, kidneys, brain, adrenal gland, spleen, and thymus.
Use
Determination of urinary steroids and of steroid conjugates in blood. Contained in some commercially available brands of Kombucha as an antioxidant & organic acid In all plants and mammals - other than guinea pigs and primatesglucuronic acid is a precursor of ascorbic acid , also known as vitamin c.
ConformationUnlike its C5 epimer iduronic acid, which may occur in a number of conformations, glucuronic acid occurs in predominantly the 4C1 conformation.
Glucuronidases
Glucuronidases are those enzymes that hydrolyze the glycosidic bond between glucuronic acid and some other compound.
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biochem/physiol Actions
In humans, glucuronic acid conjugation with steroidal compounds and drugs is an important step in phase II metabolic reactions. This influences the biotransformation process of the compound.
Purification Methods
Crystallise the acid from EtOH or EtOAc, wash it with MeOH and dry it in vacuo to give the “ ” form. Heating converts it to the lactone (see below). The sodium salt monohydrate [207300-70-7] M 234.1 has m ~136-138o(dec) [] D 20 +21o (c 2, H2O after 2hours). [Sutter & Reichstein Helv Chim Acta 21 1210 1938, Beilstein 3 H 886, 3 IV 1997.]
Properties of D-Glucuronic acid
| Melting point: | 159-161 °C (lit.) |
| Boiling point: | 250.56°C (rough estimate) |
| alpha | [α]D20 +35.0~+39.0゜(c=6,H2O) |
| Density | 1.4301 (rough estimate) |
| refractive index | 36 ° (C=6, H2O) |
| storage temp. | -20°C |
| solubility | H2O: soluble100mg/mL, clear to slightly hazy |
| form | Crystalline Powder |
| pka | pKa 3.18(H2O
t=20.0) (Uncertain) |
| color | White to off-white |
| Water Solubility | Soluble in water. |
| Merck | 14,4465 |
| BRN | 1727083 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 6556-12-3(CAS DataBase Reference) |
| EPA Substance Registry System | D-Glucuronic acid (6556-12-3) |
Safety information for D-Glucuronic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for D-Glucuronic acid
| InChIKey | LCUVNMXNGSEBHT-RJOKPDHXSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran




You may like
-
 D-Glucuronic acid CAS 6556-12-3View Details
D-Glucuronic acid CAS 6556-12-3View Details
6556-12-3 -
 D-Glucuronic acid 95% CAS 6556-12-3View Details
D-Glucuronic acid 95% CAS 6556-12-3View Details
6556-12-3 -
 D-Glucuronic Acid extrapure CAS 6556-12-3View Details
D-Glucuronic Acid extrapure CAS 6556-12-3View Details
6556-12-3 -
 D-Glucuronic Acid CAS 6556-12-3View Details
D-Glucuronic Acid CAS 6556-12-3View Details
6556-12-3 -
 D-Glucuronic acid CAS 6556-12-3View Details
D-Glucuronic acid CAS 6556-12-3View Details
6556-12-3 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
