(2R,3R,4S,5S,6R)-4-Amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxy-cyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol
- CAS NO.:34493-98-6
- Empirical Formula: C18H37N5O8
- Molecular Weight: 451.52
- MDL number: MFCD00210275
- EINECS: 252-064-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-19 15:37:51
![(2R,3R,4S,5S,6R)-4-Amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxy-cyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol Structural](https://img.chemicalbook.in/CAS/GIF/34493-98-6.gif)
What is (2R,3R,4S,5S,6R)-4-Amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxy-cyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol?
Description
Dibekacin was synthesized in 1967 by Umezawa et al. by the removal of the 3 - and 4 -hydroxyl groups of kanamycin B. Studies by the same workers on the mechanisms of bacterial resistance to kanamycin-group antibiotics preceded the discovery. Dibekacin shows excellent activity, as expected, against a variety of bacteria, including kanamycin-resistant strains. It shows higher activity than kanamycin against Pseudomonas aeruginosa, Proteus, and other pathogens.
Originator
Panimycin,Meiji Seika,Meiji Seika,1975
The Uses of (2R,3R,4S,5S,6R)-4-Amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxy-cyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol
Dideoxykanamycin B is an antibacterial compound.
Background
Dibekacin is an aminoglycoside antibiotic marketed in Japan .
Definition
ChEBI: A kanamycin that is kanamycin B lacking the 3- and 4-hydroxy groups on the 2,6-diaminosugar ring.
Manufacturing Process
Penta-N-benzyloxycarbonyl-2"-O-benzylsulfonyl-3',4'-dideoxy-3'-enokanamycin B (61 mg) was dissolved in about 18 ml of liquid ammonia at -
50°C, followed by addition of about 120 mg of metal sodium. The mixture was
gently stirred at -50°C for 1 hour, followed by addition of methanol to
consume up the excess of the metal sodium. The reaction mixture was
allowed to slowly raise up to ambient temperature while permitting the
ammonia to evaporate. The residue so obtained was dissolved in water, and
the aqueous solution was admixed with 4 ml of a cation-exchange resin,
Dowex 50WX2 (H cycle) (a product of Dow Chemical Co., USA) under stirring.
The admixture comprising the resin was placed on the top of a column of 3,5
ml of the same resin. Dowex 50WX2, and the whole resin column was well
washed with water and then eluted using 1 M aqueous ammonia as the
developing solvent. The eluate was collected in fractions, and such fractions
which gave positive reaction with ninhydrin were combined together and
concentrated to dryness, affording 3',4'-dideoxy-3'-eno-kanamycin B in the
form of its monocarbonate. The yield was 23,8 mg (97%).
The product (12.1 mg) obtained in the above step was dissolved in 0.3 ml of
water, to which was then added a catalytic quantity (about 5 mg) of platinum
oxide. Hydrogenation was made with hydrogen gas at a pressure of 3.5
kg/cm2 for 1? hours. The reaction solution was filtered to remove the
catalyst, and the filtrate was concentrated to dryness, giving the desired
product 3',4'-dideoxykanamycin B in the form of its monocarbonate. The yield
was 11.5 mg (95%). [α]D25 +110° (c 1, water). The overall yield of 3',4'-
dideoxykanamycin B based on the starting kanamycin B was 57%.
Therapeutic Function
Antibacterial
Antimicrobial activity
3′,4′-Dideoxy kanamycin B. A semisynthetic aminoglycoside
closely related to the natural compound tobramycin (3′-deoxy
kanamycin B). Supplied as the sulfate.
It is active against staphylococci including methicillinresistant
strains, a wide range of enterobacteria, Acinetobacter
and Pseudomonas spp. It is also active against M. tuberculosis
and the M. avium complex (MICs 4–16 mg/L). It exhibits
the usual aminoglycoside properties of bactericidal activity
at concentrations close to the MIC and bactericidal synergy
with selected β-lactam antibiotics.
Absence of hydroxyl groups present in the parent kanamycin
B renders dibekacin resistant to phosphorylation
by APH(3′). It is also resistant to some forms of ANT(4′).
However, the type of this enzyme, ANT(4′), found in
some Gram-positive organisms modifies dibekacin at the
2″-hydroxyl group; nevertheless dibekacin has much greater
activity than tobramycin against organisms that produce the
enzyme.
A 1 mg/kg intravenous bolus dose achieves a peak plasma
concentration of around 5 mg/L. The plasma half-life is
2.3 h. Protein binding is 3–12%. It is eliminated principally
by the renal route, 75–80% of the dose appearing in
the urine in the first 24 h. Elimination is inversely related to
renal function. In patients maintained on chronic hemodialysis,
the half-life rises to 54 h between dialyses and falls to
6–7 h on dialysis.
Toxic effects are those typical of aminoglycosides with a
frequency similar to or less than those of gentamicin.
It is used for severe infections caused by susceptible microorganisms,
especially those resistant to established aminoglycosides,
but availability is limited.
Safety Profile
Poison by intraperitoneal, subcutaneous, intramuscular, and intravenous routes. Moderately toxic by ingestion. Experimental teratogenic and reproductive effects. An antibacterial agent. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
Dibekacin is synthesized from kanamycin B by the application of the Tipson- Cohen deoxygenation method after selective Nand O-protections . A modified synthetic route via the 3_x0002_,4_x0002_-epoxy compound has given a high yield of more than 40 % on an industrial scale .
Metabolism
Not Available
Properties of (2R,3R,4S,5S,6R)-4-Amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxy-cyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol
| Boiling point: | 559.28°C (rough estimate) |
| alpha | D20 +132° (c = 0.65) |
| Density | 1.3132 (rough estimate) |
| refractive index | 1.7600 (estimate) |
| pka | 13.07±0.70(Predicted) |
| CAS DataBase Reference | 34493-98-6 |
Safety information for (2R,3R,4S,5S,6R)-4-Amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxy-cyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol
Computed Descriptors for (2R,3R,4S,5S,6R)-4-Amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxy-cyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
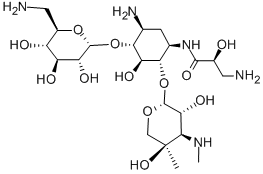



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8