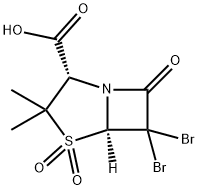
Sulbactam sodium
Synonym(s):Sulbactam sodium salt
- CAS NO.:69388-84-7
- Empirical Formula: C8H12NNaO5S
- Molecular Weight: 257.24
- MDL number: MFCD01750374
- EINECS: 273-984-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
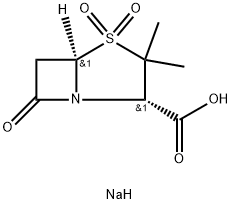
What is Sulbactam sodium?
Description
Sulbactam sodium is a parenterally-active, β-lactamase inhibitor recently introduced as a 1: 1 combination product with cefoperazone. Like clavulanic acid, the first agent of this type to b e introduced, sulbactam enhances the effectiveness of β-lactam antibiotics against resistant strains.
Chemical properties
White Solid
Originator
Pfizer (USA)
The Uses of Sulbactam sodium
A semi-synthetic β-lactamase inhibitor. It is used in combination with β-lactam antibiotics as antibacterial.
What are the applications of Application
Sulbactam sodium salt is a LACTB (β-lactamase) inhibitor that has similar characteristics as ampicillin
Definition
ChEBI: Sulbactam sodium is an organooxygen compound and an organonitrogen compound. It is functionally related to an alpha-amino acid.
Manufacturing Process
Sulbactam sodium is semi-synthetic antibiotic of penicillinic group. Start
material for it's synthesis is 6-aminopenicillanic acid. First 6-aminopenicillanic
acid was isolated in 1957 year from benzylpenicilline as resalt of treating of it
by penicillinaze. Benzylpenicilline is produced by microorganism of genus
Streptomyces.
Further, 6-aminopenicillanic acid reacted with bromine, hydrochloric acid and
NaNO2. As a result the 6,6-dibromopenicillanic acid was obtained.
6,6-Dibromopenicillanic acid was oxidized by KMnO4, to give 6,6-dibromo-1,1-The 6,6-dibromo-1,1-dioxopenicillanic acid in presence of Fe was converted to
the 1,1-dioxopenicillanic acid (sulbactam acid). The sulbactam acid was
treated by sodium 2-ethylhexanoate and crude sulbactam sodium was
obtained.
brand name
SULPERAZONE
Therapeutic Function
Beta-lactamase inhibitor
General Description
Sulbactam was synthesized by Pfizer Research Laboratories in 1977 in the course of screening for β-lactamase inhibitors. It shows strong activity against penicillinase and moderate activity against cephalosporinase. Sulbactam itself shows activity against some gramnegative bacteria but no activity against most pathogenic bacteria. The use of sulbactam in combination with cefoperazone, which is partially hydrolyzed by penicillinase, is under study along with its use as an esterified complex with ampicillin (sultamicillin) for therapy of cefoperazone-ampicillin-resistant infections.
Safety Profile
Poison by intravenous route. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx and SOx
Properties of Sulbactam sodium
| Melting point: | >230°C (dec.) |
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | Freely soluble in water, sparingly soluble in ethyl acetate, very slightly soluble in ethanol (96 per cent). It is freely soluble in dilute acids. |
| form | neat |
| form | Solid |
| color | White |
| CAS DataBase Reference | 69388-84-7(CAS DataBase Reference) |
Safety information for Sulbactam sodium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Sulbactam sodium
| InChIKey | NKZMPZCWBSWAOX-IBTYICNHSA-M |
Abamectin manufacturer
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Sulbactam sodium 95% CAS 69388-84-7View Details
Sulbactam sodium 95% CAS 69388-84-7View Details
69388-84-7 -
 Sulbactam sodium CAS 69388-84-7View Details
Sulbactam sodium CAS 69388-84-7View Details
69388-84-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4