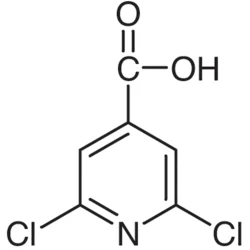
2,6-Dichloroisonicotinic acid
Synonym(s):2,6-Dichloroisonicotinic acid
- CAS NO.:5398-44-7
- Empirical Formula: C6H3Cl2NO2
- Molecular Weight: 192
- MDL number: MFCD00234147
- EINECS: 611-076-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:13:53
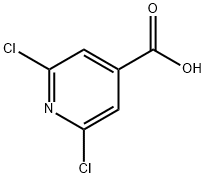
What is 2,6-Dichloroisonicotinic acid?
Chemical properties
off-white to beige or light brownish powder
The Uses of 2,6-Dichloroisonicotinic acid
2,6-Dichloroisonicotinic acid is a derivative of isonicotinic acid (I821760). Treatment of oat cultivars using 2,6-dichloroisonicotinic acid results in an increased accumulation of avenathramide. 2,6-Dichloroisonicotinic acid is a well known inducer of plant resistance and induces the transcription of ZmNAC100 transcription factor.
What are the applications of Application
2,6-Dichloropyridine-4-carboxylic acid is employed in asymmetric synthesis of (S)-(?)-acromelobic acid and biological studies of plant pathogen resistance.
Definition
ChEBI: A member of the class of pyridines that is isonicotinic acid which is substituted by chlorine at positions 2 and 6.
Agricultural Uses
The best characterized synthetic inducer of resistance, 2,6-dichloroisonicotinic acid and its methyl ester (both are referred to as INA) were the first synthetic compounds reported to activate a phenocopy of bonafide systemic acquired resistance (SAR). 2,6-dichloroisonicotinic acid was used as an abiotic resistance inducer against bacterial spot disease of tomato caused by Xanthomonas perforans. INA was found to protect cucumber, tobacco, arabidopsis, sugar beet, bean, rose, barley, and rice from several pathogens[1].
Mode of action
In tobacco, salicylic acid (SA) and 2,6-dichloroisonicotinic acid (INA) induce the same nine classes of genes (including the PR genes) that are expressed systemically after tobacco mosaic virus (TMV) infection. In contrast to many other abiotic inducers of PR gene expression and enhanced disease resistance, such as polyacrylic acid and thiamine, INA does not stimulate the accumulation of SA or its glucoside in treated tobacco plants. The strong correlation between biological activity and the ability to bind and inhibit catalase suggests that the physiological effects of INA, SA, and their active analogs are mediated by the same mechanism of action: inhibition of catalase's ability to degrade H202[2].
References
[1] S. Chandrashekar, S. Umesha. “2,6-Dichloroisonicotinic acid enhances the expression of defense genes in tomato seedlings against Xanthomonas perforans.” Physiological and Molecular Plant Pathology 86 (2014): Pages 49-56.
[2] Uwe Conrath. “Two inducers of plant defense responses, 2,6-dichloroisonicotinec acid and salicylic acid, inhibit catalase activity in tobacco.” Proceedings of the National Academy of Sciences of the United States of America 92 16 1 (1995): 7143–7.
Properties of 2,6-Dichloroisonicotinic acid
| Melting point: | 209-212 °C(lit.) |
| Boiling point: | 437.8±40.0 °C(Predicted) |
| Density | 1.612±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | soluble in Methanol |
| form | powder to crystal |
| pka | 2.63±0.10(Predicted) |
| color | White to Brown |
| Water Solubility | insoluble |
| InChI | InChI=1S/C6H3Cl2NO2/c7-4-1-3(6(10)11)2-5(8)9-4/h1-2H,(H,10,11) |
| CAS DataBase Reference | 5398-44-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Pyridinecarboxylic acid, 2,6-dichloro-(5398-44-7) |
Safety information for 2,6-Dichloroisonicotinic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,6-Dichloroisonicotinic acid
| InChIKey | SQSYNRCXIZHKAI-UHFFFAOYSA-N |
| SMILES | C1(Cl)=NC(Cl)=CC(C(O)=O)=C1 |
2,6-Dichloroisonicotinic acid manufacturer
RVR Labs Pvt Ltd
SAKEM LLP
Sarex Overseas Div of Saraf Chemicals Pvt. Ltd.
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



![2-[(4-CHLOROBENZYL)THIO]-4-(([(2,6-DICHLOROISONICOTINOYL)OXY]IMINO)METHYL)PYRIMIDINE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB9472978.gif)

You may like
-
 5398-44-7 99%View Details
5398-44-7 99%View Details
5398-44-7 -
 2,6-Dichloroisonicotinic acid 98%View Details
2,6-Dichloroisonicotinic acid 98%View Details
5398-44-7 -
 2,6-Dichloroisonicotinic Acid CAS 5398-44-7View Details
2,6-Dichloroisonicotinic Acid CAS 5398-44-7View Details
5398-44-7 -
 2,6-Dichloropyridine-4-carboxylic acid CAS 5398-44-7View Details
2,6-Dichloropyridine-4-carboxylic acid CAS 5398-44-7View Details
5398-44-7 -
 2,6-Dichloroisonicotinic Acid 98%View Details
2,6-Dichloroisonicotinic Acid 98%View Details
5398-44-7 -
 2 6 Dichloroisonicotinic AcidView Details
2 6 Dichloroisonicotinic AcidView Details
5398-44-7 -
 2,6-Dichloroisonicotinic acid (2,6-Dichloropyridine-4-carboxylic acid)View Details
2,6-Dichloroisonicotinic acid (2,6-Dichloropyridine-4-carboxylic acid)View Details
5398-44-7 -
 2,6-Dichloroisonicotinicacid 5398-44-7 98%View Details
2,6-Dichloroisonicotinicacid 5398-44-7 98%View Details
5398-44-7
