2,6-Di-tert-butylphenol
- CAS NO.:128-39-2
- Empirical Formula: C14H22O
- Molecular Weight: 206.32
- MDL number: MFCD00008820
- EINECS: 204-884-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-29 17:45:46
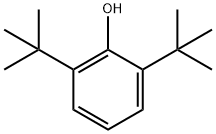
What is 2,6-Di-tert-butylphenol?
Description
2,6-Di-tert-butylphenol (2,6-DTBP) serves as the starting material in the production of the most efficient phenolic antioxidants known today and also for the synthesis of 4,4′-dihydroxybiphenyl. The compound is usually synthesised by the alkylation of phenol or of 2-tert-butylphenol (2-TBP) with isobutene. In the presence of aluminium tris-(phenolate) catalyst temperatures between 100 and 125 °C and at pressures up to 25 bar have been employed[1].
Chemical properties
white solid
The Uses of 2,6-Di-tert-butylphenol
Antioxidant for Gasoline, Jet Fuels, and Electrical Insulating Oils
The Uses of 2,6-Di-tert-butylphenol
Antioxidant Intermediate, Pharmaceuticals
The Uses of 2,6-Di-tert-butylphenol
Widely used as antioxidant in fuels, lubricants and polymers. Employed as a synthetic intermediate for the production of higher molecular weight phenolic antioxidants. Used as an oxidation inhibitor and stabilizer (e.g. for fuel, oil and gasoline) and also used in plastics and rubber. Also applied as an intermediate and an antioxidant in aviation gasoline.
Definition
ChEBI: 2,6-di-tert-butylphenol is a member of the class of phenols carrying two tert-butyl substituents at positions 2 and 6. It has a role as an antioxidant. It is a member of phenols and an alkylbenzene.
General Description
Odorless colorless to light yellow solid or liquid. Floats on water. Freezing point is 97°F.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phenols, such as 2,6-Di-tert-butylphenol, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid.
Health Hazard
Irritates eyes and (on prolonged contact) skin. Ingestion causes irritation of mouth and stomach.
Flammability and Explosibility
Non flammable
Purification Methods
Crystallise the phenol from aqueous EtOH or n-hexane. [Beilstein 6 III 2061.]
References
[1] Küpper, Friedrich-Wilhelm. “A new mechanism — key for an improved synthesis of 2,6-di-tert-butylphenol.” Applied Catalysis A: General 264 2 (2004): Pages 253-262.
Properties of 2,6-Di-tert-butylphenol
| Melting point: | 34-37 °C(lit.) |
| Boiling point: | 253 °C(lit.) |
| Density | 0.91 |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| refractive index | 1.5312 |
| Flash point: | 245 °F |
| storage temp. | Sealed in dry,2-8°C |
| solubility | 0.003g/l |
| form | Crystalline Solid |
| pka | 12.16±0.40(Predicted) |
| color | White to light yellow |
| Water Solubility | insoluble |
| FreezingPoint | 35.0 to 37.0 ℃ |
| BRN | 1841887 |
| Stability: | Stable. Incompatible with acid chlorides, acid anhydrides, bases, brass, copper, copper alloys, oxidizing agents. |
| CAS DataBase Reference | 128-39-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 2,6-bis(1,1-dimethylethyl)-(128-39-2) |
| EPA Substance Registry System | 2,6-Di-tert-butylphenol (128-39-2) |
Safety information for 2,6-Di-tert-butylphenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for 2,6-Di-tert-butylphenol
2,6-Di-tert-butylphenol manufacturer
JSK Chemicals
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 2,6-Ditertiary Butyl Phenol 99%View Details
2,6-Ditertiary Butyl Phenol 99%View Details
128-39-2 -
 2,6-Ditertiary Butyl Phenol 128-39-2 98%View Details
2,6-Ditertiary Butyl Phenol 128-39-2 98%View Details
128-39-2 -
 128-39-2 2,6-Di-tert-butylphenol, 98% 99%View Details
128-39-2 2,6-Di-tert-butylphenol, 98% 99%View Details
128-39-2 -
 2,6-Di-tert-butylphenol CAS 128-39-2View Details
2,6-Di-tert-butylphenol CAS 128-39-2View Details
128-39-2 -
 2,6-Di-tert-butylphenol CAS 128-39-2View Details
2,6-Di-tert-butylphenol CAS 128-39-2View Details
128-39-2 -
 2,6-Di-tert-butylphenol CAS 128-39-2View Details
2,6-Di-tert-butylphenol CAS 128-39-2View Details
128-39-2 -
 2 6 Di Tert Butylphenol, Purity: 99.5, Packaging Size: Bulk And DrumView Details
2 6 Di Tert Butylphenol, Purity: 99.5, Packaging Size: Bulk And DrumView Details
128-39-2 -
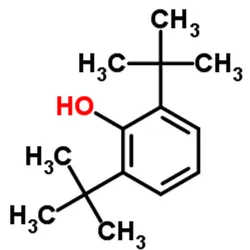 2,6 Di Tert Butylphenol (2,6-DTBP)View Details
2,6 Di Tert Butylphenol (2,6-DTBP)View Details
128-39-2
