2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane
Synonym(s):2,5-Bis(tert-butylperoxy)-2,5-dimethylhexane;2,5-Bis(tert-butylperoxy)-2,5-dimethylhexane, blend with calcium carbonate and silica
- CAS NO.:78-63-7
- Empirical Formula: C16H34O4
- Molecular Weight: 290.44
- MDL number: MFCD00053715
- EINECS: 201-128-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-09 21:58:15
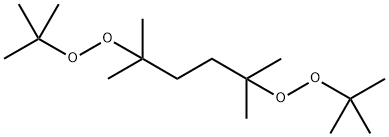
What is 2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane?
Chemical properties
colourless to light yellow liquid
The Uses of 2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane
Polymerization initiator
The Uses of 2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane
Initiates degradation of polypropylene, monomer scavenger in styrene polymerization. Free radical polymerization initiator.
What are the applications of Application
2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane is an efficient peroxide for the degradation of polypropylene (CR-PP) in the temperature range of 200-250°C. It can also be used as chaser catalyst to reduce residual monomer in the mass polymerization of styrene. It is used for the crosslinking of natural and synthetic rubber, as well as thermoplastic polyolefins: Rubber compounds containing Trigonox 101 have excellent scorch safety. Safe processing temperature: 135°C(rheometer ts2 > 20 minutes). Typical crosslinking temperature: 175 ℃ (rheometer t90 about 12 minutes).
General Description
2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane, also known as trigonox 101, is a clear, yellow liquid. (NTP, 1992)also for storage and transport mixed with inert solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Peroxides, such as 2,5-DIMETHYL-2,5-DI-(TERT-BUTYLPEROXY)HEXANE, are good oxidizing agents. Organic compounds can ignite on contact with concentrated peroxides. Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively with peroxides. There are few chemical classes that do not at least produce heat when mixed with peroxides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions of peroxides (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion. Solutions of peroxides often become explosive when evaporated to dryness or near-dryness. Danger of explosion when dry. May explode from heat, shock, friction or contamination. May ignite combustibles (wood, paper, oil, clothing, etc.). May be ignited by heat, sparks or flames.
Flammability and Explosibility
Flammable
Safety Profile
Moderately toxic by intraperitoneal route. Combustible when exposed to heat, flames, or reducing agents. To fight fire, use water spray, foam, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. Used in the polymerization of styrene and in cross-linking of various grades of polyethylene. See also PEROXIDES, ORGANIC.
Potential Exposure
It is used as a catalyst in making polyethylene, polystyrene, and polyester resins.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Color Code—Yellow Stripe: Reactivity Hazard;Store separately in an area isolated from flammables, combustibles, or other yellow-coded materials. Prior to workingwith this chemical you should be trained on its proper handling and storage. Store in tightly closed containers in acool, well-ventilated area away from strong oxidizers (suchas chlorine, bromine, and fluorine), strong acids (such ashydrochloric, sulfuric, and nitric), and combustibles.Sources of ignition, such as smoking and open flames, areprohibited where 2,5-dimethyl-2,5-di(tert-butyl peroxy)hexane is used, handled, or stored in a manner that could createa potential fire or explosion hazard. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of 2,5-dimethyl-2,5-di(tert-butyl peroxy)hexane.See OSHA Standard 1910.104 and NFPA 43A Code for theStorage of Liquid and Solid Oxidizers for detailed handlingand storage regulations.
Shipping
This compound requires a shipping label of“ORGANIC PEROXIDE.” It falls in Hazard Class 5.2 andPacking Group II.
Incompatibilities
Unstable organic peroxide. Strong oxidizer; potentially explosive. Forms explosive mixture withair. Reacts with reducing agents, strong oxidizers, strongacids, combustible materials.
Properties of 2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane
| Melting point: | 6 °C |
| Boiling point: | 55-57 °C7 mm Hg(lit.) |
| Density | 0.877 g/mL at 25 °C(lit.) |
| vapor pressure | 0.002Pa at 20℃ |
| refractive index | n |
| Flash point: | 149 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Soluble), Methanol (Sparingly) |
| form | Oil |
| color | Colourless |
| Water Solubility | immiscible |
| Stability: | Unstable. May contain an inhibitor. Incompatible with strong oxidizing agents, acids, reducing agents, organic materials, powdered metals. |
| CAS DataBase Reference | 78-63-7(CAS DataBase Reference) |
| EPA Substance Registry System | 2,5-Dimethyl-2,5-di-(tert-butylperoxy)hexane (78-63-7) |
Safety information for 2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H242:Self-reactive substances and mixtures; and Organic peroxides H315:Skin corrosion/irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P235:Keep cool. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P370+P378:In case of fire: Use … for extinction. P410:Protect from sunlight. |
Computed Descriptors for 2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4