2,4-Dimethylphenol
Synonym(s):2,4-Dimethylphenol;2,4-DMP, asym.-m-Xylenol;4-Hydroxy-m-xylene;asym.-m-Xylenol
- CAS NO.:105-67-9
- Empirical Formula: C8H10O
- Molecular Weight: 122.16
- MDL number: MFCD00002233
- EINECS: 203-321-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:55
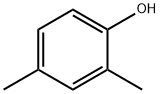
What is 2,4-Dimethylphenol?
Description
2,4-DMP is a combustible, colorless, crystalline solid. The 2,4-isomer is 1 of 5 isomers of this formula.Molecular weight= 122.18; Boiling point 212℃; Freezing/Melting point = 2728℃; Flash point = 110℃. HazardIdentification (based on NFPA-704 M Rating System):Health 1, Flammability 2, Reactivity 0. Soluble in water.
Chemical properties
2,4-Dimethylphenol is a clear colorless to yellow liquid or needle crystals. miscible with alcohol and ether, slightly soluble in water.It has a moderate mammalian oral toxicity and is a recognised irritant.
Physical properties
Colorless solid, slowly turning brown on exposure to air.
The Uses of 2,4-Dimethylphenol
2,4-dimethylphenol is a fungicide and disinfectant with a variety of agricultural uses. It is also used in making wetting agent; dyestuffs; phenolic antioxidants; pharmaceuticals; rubber chemicals; lubricant ; gasoline additive and plasticizers.
The Uses of 2,4-Dimethylphenol
It is used as a perfuming agent in cosmetic industry. Also used in the production of high-viscosity phosphate esters, as a feedstock for hindered phenol antioxidant and specialty modified phenolic resin manufacture.
Definition
ChEBI: 2,4-xylenol is a member of the class of phenols that phenol substituted by methyl groups at positions 2 and 4. It has a role as a disinfectant and a volatile oil component. It is a member of phenols and an aromatic fungicide. It derives from a hydride of a m-xylene.
Preparation
synthesis of 2,4-dimethylphenol: 2,4-Dimethylphenol is obtained by sulfonation, salting out, alkali melting and acidification of m-xylene.
General Description
2,4-dimethylphenol appears as colorless crystals or clear, dark amber liquid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2,4-Dimethylphenol is a very weak acid (pKa = 10.6) . Incompatible with acid chlorides, acid anhydrides, bases and oxidizing agents. Corrodes steel, brass, copper and copper alloys .
Health Hazard
2,4-Dimethylphenol is expected
to be an irritant of the eyes, mucous membranes,
and skin, by analogy to other phenols.
The oral LD50 for rats was 3.2 g/kg; the
dermal LD50 in mice was 1.04 g/kg.
Fire Hazard
2,4-Dimethylphenol is probably combustible.
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion and skin contact. Questionable carcinogen with experimental carcinogenic data. When heated to decomposition it emits acrid smoke and irritating fumes. See also other xylenol entries.
Potential Exposure
AgriculturalChemical; Tumorigen, Drug. 2,4-DMP finds use commercially as an important chemical feedstock or constituent forthe manufacture of a wide range of commercial productsfor industry and agriculture. 2,4-Dimethylphenol is used inthe manufacture of phenolic antioxidants, disinfectants, solvents, pharmaceuticals, insecticides, fungicides, plasticizers,rubber chemicals, polyphenylene oxide wetting agents, anddyestuffs; and is an additive or constituent of lubricants,gasolines, and cresylic acid. 2,4-Dimethylphenol (2,4-DMP)is a naturally occurring substituted phenol derived from thecresol fraction of petroleum or coal tars by fractional distillation and extraction with aqueous alkaline solutions. It isthe cresylic acid or tar acid fraction of coal tar. Workersinvolved in the fractionation and distillation of petroleum orcoal, and coal tar products comprise one group at risk.Workers who are intermittently exposed to certain commercial degreasing agents containing cresol may also be at risk.Cigarette and marijuana smoking groups and those exposedto cigarette smoke inhale microgram quantities of 2,4-demthylphenol.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If convulsions are not present, give a glass ortwo of water or milk to dilute the substance. Assure that theperson’s airway is unobstructed and contact a hospital orpoison center immediately for advice on whether or not toinduce vomiting
Carcinogenicity
2,4-Dimethylphenol was tested for mutagenicity
in the Salmonella microsome preincubation
assay using the standard protocol of the
National Toxicology Program and five strains
of Salmonella; results were negative.
The ACGIH has not established a threshold
limit value (TLV) for 2,4-dimethylphenol.
Source
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for 24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method 625. Average 2,4-dimethylphenol concentrations reported in water-soluble fractions of unleaded gasoline, kerosene, and diesel fuel were 50, 99, and 108 μg/L, respectively. 2,4-Dichlorophenol may also enter groundwater by leaching from coal tar, asphalt runoff, plastics, and pesticides (quoted, Verschueren, 1983).
Environmental Fate
Biological. When 2,4-dimethylphenol was statically incubated in the dark at 25 °C with yeast
extract and settled domestic wastewater inoculum, significant biodegradation with rapid
adaptation was observed. At concentrations of 5 and 10 mg/L, 100 and 99% biodegradation,
respectively, were observed after 7 d (Tabak et al., 1981).
Photolytic. 2,4-Dimethylphenol absorbs UV light at a maximum wavelength of 277 nm
(Dohnal and Fenclová, 1995).
Chemical/Physical. Wet oxidation of 2,4-dimethylphenol at 320 °C yielded formic and acetic
acids (Randall and Knopp, 1980). 2,4-Dimethylphenol will not hydrolyze because there is no
hydrolyzable functional group (Kollig, 1993).
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from oxidizers and sources of ignition
Shipping
This compound requires a shipping label of“POISONOUS/TOXIC MATERIALS” for xylenols. It fallsin Hazard Class 6.1 and Packing Group II.
Properties of 2,4-Dimethylphenol
| Melting point: | 22-23 °C(lit.) |
| Boiling point: | 211-212 °C(lit.) |
| Density | 1.011 g/mL at 25 °C(lit.) |
| vapor pressure | 0.1 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 205 °F |
| storage temp. | Store below +30°C. |
| solubility | 5.0g/l |
| form | Liquid |
| pka | pK1:10.58 (25°C) |
| color | Clear colorless to yellow |
| Odor | at 100.00 %. weak smoky roasted dark |
| explosive limit | 1.4%(V) |
| Water Solubility | 0.5 g/100 mL (25 ºC) |
| Merck | 14,10082 |
| BRN | 636244 |
| Henry's Law Constant | 42.8(x 10-6 atm?m3/mol) at 75.9 °C, 74.0 at 88.7 °C, 113.0 at 98.5 °C (VLE circulation still-UV spectrophotometry,
Dohnal and Fenclová, 1995) |
| CAS DataBase Reference | 105-67-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 2,4-dimethyl-(105-67-9) |
| EPA Substance Registry System | 2,4-Dimethylphenol (105-67-9) |
Safety information for 2,4-Dimethylphenol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,4-Dimethylphenol
| InChIKey | KUFFULVDNCHOFZ-UHFFFAOYSA-N |
2,4-Dimethylphenol manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
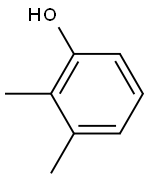

You may like
-
 105-67-9 99%View Details
105-67-9 99%View Details
105-67-9 -
 2,4-Xylenol 98%View Details
2,4-Xylenol 98%View Details
105-67-9 -
 2,4-Xylenol 105-67-9 99%View Details
2,4-Xylenol 105-67-9 99%View Details
105-67-9 -
 2,4-Dimethylphenol, 95% 105-67-9 99%View Details
2,4-Dimethylphenol, 95% 105-67-9 99%View Details
105-67-9 -
 2,4-Dimethylphenol CAS 105-67-9View Details
2,4-Dimethylphenol CAS 105-67-9View Details
105-67-9 -
 2,4-Dimethylphenol CAS 105-67-9View Details
2,4-Dimethylphenol CAS 105-67-9View Details
105-67-9 -
 2,4-Dimethylphenol CAS 105-67-9View Details
2,4-Dimethylphenol CAS 105-67-9View Details
105-67-9 -
 2,4-Xylenol CAS No.: 105-67-9View Details
2,4-Xylenol CAS No.: 105-67-9View Details
105-67-9
