2,2,2-Trifluoroethanol
Synonym(s):2,2,2-Trifluoroethanol;TFEA;transcription factor binding to IGHM enhancer 3;Transcription factor for IgH enhancer;Trifluoroethyl alcohol
- CAS NO.:75-89-8
- Empirical Formula: C2H3F3O
- Molecular Weight: 100.04
- MDL number: MFCD00004672
- EINECS: 200-913-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
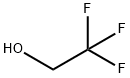
What is 2,2,2-Trifluoroethanol?
Chemical properties
Colorless liquid
The Uses of 2,2,2-Trifluoroethanol
2,2,2-Trifluoroethanol (Trifluoroethyl alcohol, TFE) is used to study the conformational states of proteins and the folding refolding processes of proteins.
The Uses of 2,2,2-Trifluoroethanol
In synthesis of medical anaesthetics, pharmaceuticals, and agrochemicals; in polymerizations. Protein denaturant; stabilizes peptide structures. Cleaning solvent; eluent in HPLC separations; working fluid in Rankine heat cycle systems. Environmentally friendly alternative to CFCs.
The Uses of 2,2,2-Trifluoroethanol
Trifluoroethanol serves as a solvent and a raw material in organic chemistry and biology. TFE is a solvent of choice for hydrogen peroxide-mediated oxidations of sulfides. Trifluoroethanol acts as a protein denaturant. It is used in the manufacture of certain pharmaceuticals and drug substances. The drug fluromer, which is 2,2,2-trifluoro-1-vinyloxyethane, is the vinyl ether of trifluorethanol. It is an effective solvent for peptides and proteins, and used for NMR-based protein folding studies, and in the manufacture of nylon. As a source of the trifluoromethyl group, it is employed in several organic reactions, for example in Still-Gennari modification of Horner-Wadsworth-Emmons reaction (HWE) reaction.
What are the applications of Application
2,2,2-Trifluoroethanol is an acidic alcohol that forms stable complexes with heterocycles
Definition
ChEBI: 2,2,2-trifluoroethanol is a fluoroalcohol. It is functionally related to an ethanol.
Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by inhalation and skin contact. Experimental reproductive effects. A severe skin and eye irritant. When heated to decomposition it emits toxic fumes of F-.
Purification Methods
Dry it with CaSO4 and a little NaHCO3 (to remove traces of acid) and distil it. Highly TOXIC vapour. [Beilstein 1 IV 1370.]
Properties of 2,2,2-Trifluoroethanol
| Melting point: | −44 °C(lit.) |
| Boiling point: | 77-80 °C(lit.) |
| Density | 1.391 g/mL at 20 °C |
| vapor density | 3.5 (vs air) |
| vapor pressure | 70 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 85 °F |
| storage temp. | Store below +30°C. |
| solubility | soluble in Chloroform, Methanol |
| pka | 12.4(at 25℃) |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 1.373 |
| PH Range | 5-7 (10% aq soln) |
| Odor | Alcoholic odour |
| explosive limit | 8.4-28.8%(V) |
| Water Solubility | Miscible with water, ethers, ketones, alcohols and chloroform. |
| λmax | λ: 260 nm Amax: ≤0.03 λ: 280 nm Amax: ≤0.02 |
| Merck | 14,9682 |
| BRN | 1733203 |
| Dielectric constant | 8.5500000000000007 |
| Stability: | Stable. Flammable - note wide explosion limits. Moisture sensitive. Incompatible with strong oxidizing agents, strong acids, sodium, potassium. |
| CAS DataBase Reference | 75-89-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethanol, 2,2,2-trifluoro-(75-89-8) |
| EPA Substance Registry System | 2,2,2-Trifluoroethanol (75-89-8) |
Safety information for 2,2,2-Trifluoroethanol
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H318:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,2,2-Trifluoroethanol
| InChIKey | RHQDFWAXVIIEBN-UHFFFAOYSA-N |
2,2,2-Trifluoroethanol manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 2,2,2 TRIFLUORO ETHANOL 99%View Details
2,2,2 TRIFLUORO ETHANOL 99%View Details -
 75-89-8 2,2,2-Trifluoroethanol, 99% 99%View Details
75-89-8 2,2,2-Trifluoroethanol, 99% 99%View Details
75-89-8 -
 2,2,2-Trifluoroethanol CAS 75-89-8View Details
2,2,2-Trifluoroethanol CAS 75-89-8View Details
75-89-8 -
![2,2,2-Trifluoroethanol [for HPLC] CAS 75-89-8](https://img.chemicalbook.in//Content/image/CP5.jpg) 2,2,2-Trifluoroethanol [for HPLC] CAS 75-89-8View Details
2,2,2-Trifluoroethanol [for HPLC] CAS 75-89-8View Details
75-89-8 -
 2,2,2-TRIFLUOROETHANOL For Synthesis CAS 75-89-8View Details
2,2,2-TRIFLUOROETHANOL For Synthesis CAS 75-89-8View Details
75-89-8 -
 Anti-TFE3 (N-terminal) antibody produced in goat CASView Details
Anti-TFE3 (N-terminal) antibody produced in goat CASView Details -
 Trifluoroethanol Liquid Chemical, Grade Standard: Technical Grade, LooseView Details
Trifluoroethanol Liquid Chemical, Grade Standard: Technical Grade, LooseView Details
75-89-8 -
 2 2 2 Trifluoroethanol Liquid ChemicalView Details
2 2 2 Trifluoroethanol Liquid ChemicalView Details
75-89-8
