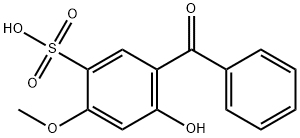
2,2'-Dihydroxy-4-methoxybenzophenone
Synonym(s):2,2′-Dihydroxy-4-methoxybenzophenone;Benzophenone-8;Dioxybenzone
- CAS NO.:131-53-3
- Empirical Formula: C14H12O4
- Molecular Weight: 244.24
- MDL number: MFCD00002218
- EINECS: 205-026-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
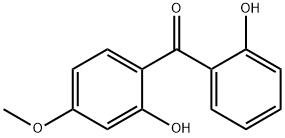
What is 2,2'-Dihydroxy-4-methoxybenzophenone ?
Absorption
Dioxybenzone is a derivative of benzophenone. In monkeys, percutaneous absorption of benzophenone was observed . Other derivatives of benzophenone are capable of crossing the skin via direct penetration through the intercellular laminae of the stratum corneum (SC) or by passive diffusion by high-concentration gradient into the systemic circulation, where they are transported to different tissues including liver and brain .
Toxicity
No toxicokinetic data available.
Chemical properties
Light yellow powder. Freezing point 68℃, boiling point 170-175℃(0.133kPa), relative density 1.382(25℃).Solubility at 25℃(g/100g solvent): benzene 46.6, n-hexane 2.3, 95% ethanol 21.4, carbon tetrachloride 22.2, methylethyl ketone 55.3, plasticiser DOP 31.1, insoluble in water.
The Uses of 2,2'-Dihydroxy-4-methoxybenzophenone
ultraviolet screen
The Uses of 2,2'-Dihydroxy-4-methoxybenzophenone
benzophenone-8 (dioxybenzone) is an FDA-approved sunscreen chemical with uVA-absorption capabilities. It also can protect product degradation arising from uV-light exposure. It has an approved usage level of 3 percent in the united States.
What are the applications of Application
Dioxybenzone is a compound used in sunscreen formulations to block out UVB and UVA harmful rays. Broad-spectrum sunscreens often contain a number of chemical ingredients that absorb UVA and UVB radiation. Many sunscreens contain UVA-absorbing avobenzone or a benzophenone (such as dioxybenzone, oxybenzone, or sulisobenzone), in addition to UVB-absorbing chemical ingredients (some of which also contribute to UVA protection).
Background
Dioxybenzone, or benzophenone-8, is an organic compound derived from Benzophenone that is used as a sunscreen agent. It absorbed UV-B and UV-AII rays. Dioxybenzone is an approved sunscreen ingredient in concentrations up to 3% .
Indications
Indicated for use as an active sunscreen agent.
Preparation
preparation by condensation of salicylic acid and m-methoxyphenol.
Definition
ChEBI: 2,2'-Dihydroxy-4-methoxybenzophenone is a member of benzophenones.
General Description
Dioxybenzone, also known as benzophenone-8, is an organic compound and a derivative of benzophenone used commercially in sunscreens. It absorbed UV-B and UV-AII rays. Dioxybenzone is an approved sunscreen ingredient in concentrations up to 3%.
Reactivity Profile
An alcohol and a ketone. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Fire Hazard
Flash point data for 2,2'-Dihydroxy-4-methoxybenzophenone are not available. 2,2'-Dihydroxy-4-methoxybenzophenone is probably combustible.
Pharmacokinetics
Dioxybenzone is a sunscreen agent and chemical UV filter that absorbs UV-B rays and UV-AII rays to limit their penetration into human skin. In a screening protocol consisting of the in vitro EBV-EA activation assay followed by the in vivo confirmation test in the two-stage mouse skin cancer model utilizing NOR-1 as inducer and TPA as promoter of tumour, dioxybenzone exhibited a significant chemopreventive activity against mouse skin carcinogenesis which correlated with their antioxidant potency . There is some evidence that suggests some benzophenones and their hydroxylated metabolites act as weak estrogens in the environment; however similar effect of dioxybenzone has not been established .
Metabolism
No pharmacokinetic data available.
Properties of 2,2'-Dihydroxy-4-methoxybenzophenone
| Melting point: | 73-75 °C(lit.) |
| Boiling point: | 170-175 °C1 mm Hg(lit.) |
| Density | 1.2379 (rough estimate) |
| vapor pressure | 0-0Pa at 20-25℃ |
| refractive index | 1.5389 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 7.11±0.35(Predicted) |
| form | Solid |
| color | Off-White to Yellow |
| Merck | 14,3303 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 131-53-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Dioxybenzone(131-53-3) |
| EPA Substance Registry System | Dioxybenzone (131-53-3) |
Safety information for 2,2'-Dihydroxy-4-methoxybenzophenone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,2'-Dihydroxy-4-methoxybenzophenone
| InChIKey | MEZZCSHVIGVWFI-UHFFFAOYSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


![Poly[N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-hexanediamine-co-2,4-dichloro-6-morpholino-1,3,5-triazine]](https://img.chemicalbook.in/CAS/GIF/82451-48-7.gif)
You may like
-
 2,2'-Dihydroxy-4-methoxybenzophenone CAS 131-53-3View Details
2,2'-Dihydroxy-4-methoxybenzophenone CAS 131-53-3View Details
131-53-3 -
 Dioxybenzone 98.00% CAS 131-53-3View Details
Dioxybenzone 98.00% CAS 131-53-3View Details
131-53-3 -
 Dioxybenzone CAS 131-53-3View Details
Dioxybenzone CAS 131-53-3View Details
131-53-3 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
