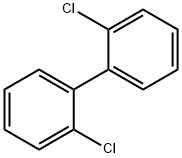
2,2'-dichlorobenzidine
- CAS NO.:84-68-4
- Empirical Formula: C12H10Cl2N2
- Molecular Weight: 253.13
- MDL number: MFCD00209459
- EINECS: 201-552-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-29 18:36:46

What is 2,2'-dichlorobenzidine?
Chemical properties
3,3' -Dichlorobenzidine is barely discolored on exposure to air and is chemically resistant to water. Oxidizing agents, such as bromine water, potassium dichromate, and iron(III) chloride, cause the formation of a green color. With gold, the green color is still detectable at a dilution of 1 : 5000000. N-Acetyl-3,3' -dichlorobenzidine and N,N' - diacetyl-3,3' -dichlorobenzidine form when 3,3' - dichlorobenzidine is treated with acetic anhydride in dilute alcohol. The tetrazotizing behavior is similar to that of benzidine and o-tolidine.
The Uses of 2,2'-dichlorobenzidine
3,3' -Dichlorobenzidine was introduced about 1932 and is now the most important diphenyl base. It is used as the starting material for pigments with yellow and red shades. These are used for coloring printing inks, paints, plastics, and rubbers. The important diarylide yellow pigments, which are incorrectly known as benzidine yellows, are formed by the combination of 3,3' - dichlorobenzidine with acetic acid arylides. 3,3' - Dichlorobenzidine is also used in the production of polyurethane rubbers.
The Uses of 2,2'-dichlorobenzidine
4,4''-Diamino-2,2''-Dichlorobiphenyl is a useful reactant for the synthesis of benzidine, L-?proline, and diaminofluorene derivatives which are hepatitis C virus NS5A inhibitors.
Production Methods
2,2' -Dichlorobenzidine is made from m-nitrochlorobenzene, preferably using zinc dust and sodium hydroxide solution. It is subsequently rearranged and isolated in the form of a dihydrochloride.
Production Methods
3,3' -Dichlorobenzidine is made from o-nitro-chlorobenzene by reduction with zinc dust and sodium hydroxide solution and subsequent rearrangement with dilute hydrochloric acid or sulfuric acid. It is assayed by titration with sodium nitrite solution in dilute hydrochloric acid and detected in human urine by colorimetry using chloramine. The detection limit is 0.1 mg/kg.
Solubility in water
It is almost insoluble in water but readily soluble in alcohol.
Purification Methods
Crystallise the benzidine from EtOH or H2O. [Beilstein 13 H 234, 13 I 66, 13 II 106, 13 III 477, 13 IV 384.]
Properties of 2,2'-dichlorobenzidine
| Melting point: | 165°C |
| Boiling point: | 398.89°C (rough estimate) |
| Density | 1.3171 (rough estimate) |
| refractive index | 1.6400 (estimate) |
| pka | 3.58±0.10(Predicted) |
Safety information for 2,2'-dichlorobenzidine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,2'-dichlorobenzidine
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran

You may like
-
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 (3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
(3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
54314-85-1 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2